Abstract
Objective
Iterative reconstruction (IR) is a noise reduction method that facilitates the synthesis of maximum intensity projection (MIP) from a larger number of slices while maintaining resolution. The present study aimed to analyze whether CT evaluation using IR and MIP is ideal for thrombus evaluation of large vessel occlusions in patients with acute ischemic stroke.
Methods
Three types of images for each patient were reconstructed and categorized into three groups: the “conventional group,” evaluated using 0.5-mm slice CT, the “MIP group,” evaluated using 0.5-mm slice CT processed with MIP, and the “IR + MIP group,” evaluated with 0.5-mm slice CT processed with IR and MIP. Noise and image quality were evaluated with noise standard deviation (Noise SD) and contrast-to-noise ratio (CNR). Three experts evaluated the thrombus edge coordinates, made a visual assessment, and compared the data with the digital subtraction angiography (DSA) of the mechanical thrombectomy.
Results
Twenty-nine patients with cerebral infarction having large vessel occlusion were included in this study. The IR + MIP group had a lower Noise SD and a statistically higher CNR, leading to more favorable image evaluations. The thrombus assessment showed no inter-rater variability in thrombus edge identification, and the visual assessment and comparison with DSA were statistically better in the IR + MIP group.
Conclusions
IR reduces noise and improves resolution. MIP in combination with IR facilitates visualization of thrombus.
Similar content being viewed by others
Introduction
The resolution of multi-slice computed tomography (CT) has improved dramatically with the development of multiple rows and technological advances. Consequently, the use of 3D reconstitution and multiple cross-sectional reconstructions from thin-slice CT data has become widespread. Thus, improving the quality of thin-slice images has become more important [1]. Noise in CT images is mainly caused by the number of X-ray photons and increases inversely with the square root of the image slice thickness. Thin-slice CT reduces the number of photons detected, which worsens the image noise. The radiation dose must change in inverse proportion to the slice thickness to maintain constant image noise for varying reconstructed slice thicknesses. For example, reducing the section thickness by 50% requires approximately twice the radiation dose to maintain the same noise level [2]. In maximum intensity projection (MIP), the voxel with the highest attenuation value on every view throughout the volume is projected onto a 2D image [3]. Reconstruction is performed using CT thin slices [4]. However, thin slices are low contrast and contain high-intensity noise; thus, the noise accumulates in MIP and the number of slices that can be synthesized using MIP is limited. Techniques to reduce noise and radiation dose are currently being developed, including automated tube current modulation and noise-reduction filters [5]. However, the effects of these techniques are limited because of rigid and thick skulls. Iterative reconstruction (IR) is a recently established innovative noise reduction method [6]. IR suppresses artifacts caused by bone and reduces noise, resulting in high-quality images using low radiation doses [5]. IR, which can be used with almost all equipment manufacturers, facilitates the synthesis of MIPs from a larger number of slices while maintaining the resolution.
In patients with stroke, thrombus diagnosis using CT has been widely investigated. Several studies focused on thrombus detection using non-contrast CT with MIP [4, 7]. We hypothesize that CT evaluation using IR and MIP is more useful for evaluating thrombus in patients with stroke. To date, thrombus information from CT has provided many insights into treatment strategies. A high-signal thrombus on CT is more likely to be rich in red blood cells [8], which leads to a high rate of recanalization [9] and short procedure time in mechanical thrombectomy [10]. Herein, we demonstrate that non-contrast CT with IR and MIP is effective in the evaluation of thrombus.
Methods
Data availability statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request and with approval from the coauthors and the hospital ethics committee.
Study population
This was a single-center prospective study. The study included consecutive patients with acute cerebral infarction caused by large vessel occlusion who were registered at the single hospital between January 2020 and January 2021. Inclusion criteria were hospitalization and eligibility for recombinant tissue plasminogen activator (rt-PA) or mechanical thrombectomy. In addition, cases with intracranial large vessel occlusion were selected. Exclusion criteria were (1) thrombus extending to extracranial, (2) recanalization with t-PA, (3) multi-vessel occlusion, and (4) patients receiving low scores from all the evaluators for the 5-point visual assessment of the thrombus described below. All patients admitted during this period were examined by neurologists and neurosurgeons and underwent CT. Cerebral infarction was usually diagnosed based on CT alone, and the indication for rt-PA was determined. Next, magnetic resonance imaging (MRI) was immediately performed and mechanical thrombectomy was performed if indicated.
The patients or their relatives provided informed consent according to ethical regulations. The hospital’s ethics committee approved the study protocol. This study is registered in the UMIN Registry, Japan (UMIN000040807).
Image acquisition and reconstruction
All patients were scanned using a multidetector CT scanner with 80 detector rows (Aquilion Prime, Canon, Japan). The standard CT protocol entailed a collimation of 0.5 mm, a tube voltage of 120 kV, and a tube current of 350 mA. Three types of images were reconstructed from the same patients and categorized into three groups as follows: the “conventional group,” evaluated using 0.5-mm slices of nonenhanced CT; the “MIP group,” evaluated using 0.5-mm slices of nonenhanced CT with MIP, and the “IR + MIP group,” evaluated using 0.5-mm slices of nonenhanced CT with IR and MIP. IR was performed using the AIDE 3D software by Canon with the standard settings. The MIP comprised ten slices. Images were evaluated on reconstructed coronal views. The coronal view is used because this view facilitates the evaluation of basilar arteries, comparison with digital subtraction angiography (DSA) because it shares the same view as that of DSA, and helps avoid overlapping M1 vessels with bone when MIP is performed. Image reconstruction was performed automatically and was completed within 30 s after imaging.
Evaluation of image noise
A comparative evaluation of the amount of noise was performed. The noise standard deviation (Noise SD) indicated the severity of image noise and could serve as part of the objective assessment of the image quality. The contrast-to-noise ratio (CNR) was used to evaluate low contrast detectability [11]. The region of interest for the Noise SD and background value for CNR calculation was the area from the intracranial internal carotid artery (ICA) to the M1 segment on the opposite side of the occluded vessel, where a sufficient area could be obtained. CNR may not match the visual assessment [12]. In this study, the objective was not to evaluate organs, but to evaluate thrombus and blood vessels, which have simple signal intensities. CNR (CNR = (signal intensity of thrombus − signal intensity of blood vessel)/Noise SD of blood vessel) was enough to distinguish between thrombus and blood vessels. All images were evaluated using the Osirix HD, version 11.0.
Thrombus evaluation
Two neurosurgeons and one neurologist with expertise in endovascular and cerebral infarction treatment evaluated the results independently. Studies show that MIP without IR is effective in detecting thrombus [4, 7]. The aim of this study was not to detect, but to visualize the entire thrombus. In cardiology studies, curved multiplanar reconstruction is used to evaluate stenotic or occlusive lesions [13]. Although the cerebral blood vessels have more flexion than coronary arteries, the bias in the procedure is expected to be significant when the vessel is straightened. Thus, we evaluated both the coordinates of the thrombus edge and the visual scoring. Clear identification of the thrombus edges is equivalent to the ability to visualize the entire length of the thrombus. Because detection is not the goal, the evaluator was informed in advance regarding the location of the thrombus as right or left, ICA, M1, M2, or BA. The evaluator was asked to indicate the proximal and distal ends of the thrombus, and the coordinates were collated. The coordinates pointed to by the evaluator were calculated by the Osirix software from the DICOM information. The position in the x, y, and z axes is displayed in millimeters scale. The distance between evaluators was calculated from the coordinates. Owing to the limited number of cases, we were concerned about the possibility of unconscious recall of thrombus features. Thus, we evaluated the results for the conventional and IR + MIP groups. The images were randomized and presented in two separate sessions at least 1 week apart. The Noise SDs and CNRs in the conventional and MIP groups were not significantly different. Compared with MIP, thin-slice CT evaluations are more widely used and more versatile [14, 15]. Moreover, the diameter of the middle cerebral artery is only 2–3 mm, suggesting that thinner slices are appropriate for assessing the thrombus edges.
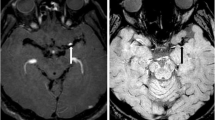
In the evaluation of the conventional group, the window level (WL) was set at 50 and the Window Width (WW) was set at 100 at first. These settings were standard for brain evaluation, and the examiners were allowed to change the settings. In the IR + MIP group, the radiodensity of the vessels was 42.91 ± 4.79 (mean, SD) Hounsfield units (HU), and the signal value of the thrombus was 61.69 ± 6.24 HU. Therefore, the WL and WW were set at 40 and 20, respectively, and the evaluators were allowed to change the settings (Fig. 1). The visual clarity of the thrombus edge was assessed using a 5-point scale (5: excellent, 4: very good, 3: good, 2: poor, and 1: non-diagnostic). When mechanical thrombectomy was performed, the homology between the location of the thrombus on the CT and that on the DSA was evaluated using a 5-point scale (5: perfect match, 4: rough match, 3: displaced more than the diameter of the occluded vessel, 2: completely different location, and 1: unable to evaluate on imaging). The proximal and distal edges of the thrombus on the DSA were obtained through contrast injection using a microcatheter and a proximal catheter which is guiding or aspiration catheters. The information obtained from this process was used to evaluate the location of the thrombus.
Computed tomography imaging with iterative reconstruction and maximum intensity projection. From left to right: axial view of nonenhanced computed tomography (CT) 0.5-mm slice, coronal view of nonenhanced CT 0.5-mm slice, slice after iterative reconstruction (IR) and maximum intensity projection (MIP), and IR + MIP slice (setting as Window Level 40, Window Width 20). Cases 1 and 2 are cases of right middle cerebral artery occlusion. The thrombus is clearer, particularly the edges, in the IR + MIP slice when the WL and WW are focused. Case 3 is a case of BA occlusion. The thrombus can be detected with nonenhanced CT; however, the noise in the blood vessel is strong and the thrombus edges are unclear
Statistical analyses
The Noise SD and CNR values are shown as medians with interquartile ranges. The Noise SD and CNR among the three groups were compared using one-way analysis of variance. The distance between the coordinates of the thrombus edge, visual clarity at the edges, and evaluation with DSA were analyzed between IR + MIP and conventional groups. Differences in the distance between the proximal and distal coordinates of the thrombus edges among the evaluators (A, B, and C) were compared using paired-sample t tests. Visual clarity at the edges and evaluation with DSA were analyzed using the Wilcoxon rank-sum test to examine the scores on conventional and IR + MIP images presented to the evaluator in a blinded and randomized setting. For all analyses, a P value of < 0.05 was considered statistically significant. Statistical analyses were conducted using the statistical package R, version 4.0.3.
Results
Thirty-five patients were admitted to the study. Three patients with extracranial occlusions, 1 patient with recanalization after admission, and 1 patient with multiple vessel occlusions were excluded from the study. In addition, one patient was excluded because all evaluators evaluated the clot as not visible on CT in the visual assessment. Thus, 29 patients were included in the study (median age, 84 years; interquartile range, 76–89 years). CT was performed 4.07 ± 4.28 min (mean ± SD) after admission, MRI was started 24.9 ± 9.95 min after CT, and 33.71 ± 31.72 min passed between the MRI and the puncture for the mechanical thrombectomy. The rt-PA was administered in 20 patients, and mechanical thrombectomies were performed in 25 patients.
Image quality evaluation
Noise SDs were significantly different among the three groups (conventional group 5.939 [5.425–6.861]; MIP group: 5.961 [5.051–6.501]; and IR + MIP group: 3.918 [3.262–4.810]; P < 0.001). No statistical differences in Noise SDs were detected between the conventional and MIP groups (P = 1). However, the Noise SDs of conventional and IR + MIP groups (P < 0.001) and MIP and IR + MIP groups (P < 0.001) were significantly different (Fig. 2). CNRs were also significantly different (conventional group 3.345 [2.412–4.368]; MIP group: 2.824 [2.330–3.628]; and IR + MIP group 4.159 [3.262–4.981]; P = 0.02). No significant differences in CNRs in the conventional and MIP groups (P = 1) were detected. However, CNRs in the MIP and IR + MIP groups (P = 0.025) and the conventional and IR + MIP groups (P = 0.0056) were significantly different (Fig. 2). These results demonstrated that the IR + MIP group had lesser noise and higher resolution than the conventional and MIP groups.
Thrombus evaluation
In the IR + MIP group, the distance between the coordinates of the thrombus edge specified by the evaluators was much shorter than the distance in the conventional group (P < 0.001). This indicates that the thrombus edge could be identified with less variability. The radiodensity of the vessels was 42.91 ± 4.79 (mean ± SD) Hounsfield units (HU), and the signal value of the thrombus was 61.69 ± 6.24 HU in the IR + MIP group. Visual evaluations were better in the IR + MIP group than in the conventional group (P < 0.001). Furthermore, the homology of the thrombus with the DSA increased in the IR + MIP group compared with the conventional group (P < 0.001) (Table 1).
Discussion
In this study, we showed that IR reduces noise and improves resolution. Consequently, IR combined with MIP improved the visibility of the thrombus. Three diagnostic tools are used to assess and make patient treatment decisions for patients with acute stroke: CT, MRI, and transcranial Doppler or transcranial color-coded sonography [16]. Thrombus evaluation by CT angiography is the current gold standard [16]. However, the use of contrast media may cause adverse reactions, such as anaphylactic shock and contrast media nephropathy [17]. Subsequent mechanical thrombectomy requires additional contrast media, which is a serious physical burden, particularly for elderly patients. Thus, visualization of the entire length of the embolized thrombus using only non-contrast CT is valuable. Moreover, it is the simplest in terms of time and can shorten the time to treatment. Thus, it is now clear that the early administration of rt-PA and mechanical thrombectomy is associated with good patient prognosis [18, 19].
A thrombus due to intracranial middle carotid artery occlusion presents as a hyper-dense middle cerebral artery on non-contrast CT [20]. Thrombus imaging distal from M2 presents as a “Dot” sign [21]. On CT, the partial volume effect reduces the intensity of the thrombus. In addition, the continuity of the blood vessel is lost, resulting in a point-like appearance that is difficult to verify as a thrombus. However, thrombus detection by thin-slice non-contrast CT and MIP is effective [4, 7]. Using MIP, blood vessels, which are visualized as dots in a single slice, can be visualized as a single vascular structure when multiple slices are combined. Thus, thrombus assessment and verification are more effective using CT combined with MIP.
To synthesize better MIP images, several thin CT images are needed. Thinner CT slices generate more noise and thus require higher radiation doses to reduce the noise. If the noise is extremely high, the MIP images cannot be synthesized. IR can reduce noise, improve resolution, and enable MIP synthesis from multiple slices with low radiation doses. We have demonstrated that MIP created from thin-slice CT images using IR results in lower Noise SD; thus, IR reduced the noise. Moreover, CNR showed that the resolution was improved after IR. Consequently, the coordinates evaluated in the IR + MIP group were less scattered than in the conventional group, and the thrombus edges were more accurately identified. The visual evaluation was also better. These results demonstrate that the use of MIP created from thin-slice CT images after IR is an excellent method for visualizing the total length of a thrombus.
Evaluation of the entire thrombus facilitates planning treatment strategies, particularly mechanical thrombectomy. Visualization of the entire thrombus enables the selection and preparation of appropriate devices before surgery, which can save time and improve patient outcomes. Moreover, the distal ends of the thrombus can now be visualized. DSA, CT, and MR angiography are flow-related procedures, and accurate evaluation of the distal end of the thrombus is often difficult because the contrast media stagnates at the proximal end of the thrombus. By knowing the distal end of the thrombus, the selection of vessels for cannulation, the position of the microcatheter tip, and the position of the device deployment can be determined before the procedure. This saves time during the procedure and makes the procedure safer.
Noise cancellation continues to develop, including new reconstruction techniques based on deep learning using artificial intelligence [22]. In addition, the evolution and development of CT equipment, such as dual energy CT, is expected. However, it is not widely available yet. Thus, IR, which is available for general use, is valuable and can be widely used.
Limitations
This was a single-center study. Owing to the COVID-19 pandemic, the number of patients included in the study was lower than expected. A study including a larger number of patients should be considered. In this study, only one model of IR was used and other IR models should be explored. The reduction of noise facilitates the inclusion of an unlimited number of slices in the MIP; however, the optimal number of slices for MIP should be determined. In this study, the radiation dose was set slightly higher to sufficiently evaluate 0.5-mm slices. A lower dose would have made a clearer difference. One thrombus was undetectable on CT and was believed to be a white thrombus. The cases of hypo-dense occlusive thrombus were excluded in this study. Investigation of white thrombus and other non-thrombus substances, including fungus balls due to infectious endocarditis, should be investigated. CT images and DSA, which were used for evaluation, cannot be recorded simultaneously. The thrombus may migrate before treatment [23], which limits such thrombus evaluation.
Conclusions
Iterative reconstruction of non-enhanced CT images reduces noise and improves resolution, while maximum intensity projection combined with IR further enhances thrombus visualization for accurate diagnosis of acute ischemic stroke.
Abbreviations
- CT:
-
Computed tomography CNRContrast-to-noise ratio DSADigital subtraction angiography
- HU:
-
Hounsfield units
- ICA:
-
Internal carotid artery
- IR:
-
Iterative reconstruction MRIMagnetic resonance imaging
- MIP:
-
Maximum intensity projections
- rt-PA:
-
Recombinant tissue plasminogen activator
- WL:
-
Window level
- WW:
-
Window width
References
Parrish FJ (2007) Volume CT: state-of-the-art reporting. AJR Am J Roentgenol 189:528–534. https://doi.org/10.2214/AJR.07.2426
Kanal KM, Stewart BK, Kolokythas O, Shuman WP (2007) Impact of operator-selected image noise index and reconstruction slice thickness on patient radiation dose in 64-MDCT. AJR Am J Roentgenol 189:219–225. https://doi.org/10.2214/AJR.06.1524
Fishman EK, Ney DR, Heath DG, Corl FM, Horton KM, Johnson PT (2006) Volume rendering versus maximum intensity projection in CT angiography: what works best, when, and why. Radiographics 26:905–922. https://doi.org/10.1148/rg.263055186
Riedel CH, Zoubie J, Ulmer S, Gierthmuehlen J, Jansen O (2012) Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke 43:2319–2323. https://doi.org/10.1161/STROKEAHA.112.649921
Kalra MK, Maher MM, Toth TL, Schmidt B, Westerman BL, Morgan HT, Saini S (2004) Techniques and applications of automatic tube current modulation for CT. Radiology 233:649–657. https://doi.org/10.1148/radiol.2333031150
Stiller W (2018) Basics of iterative reconstruction methods in computed tomography: a vendor-independent overview. Eur J Radiol 109:147–154. https://doi.org/10.1016/j.ejrad.2018.10.025
Rosskopf J, Kloth C, Dreyhaupt J, Braun M, Schmitz BL, Graeter T (2020) Thin slices and maximum intensity projection reconstructions increase sensitivity to hyperdense middle cerebral artery sign in acute ischemic Stroke. Cerebrovasc Dis 49:437–441. https://doi.org/10.1159/000509378
Sporns PB, Hanning U, Schwindt W, Velasco A, Buerke B, Cnyrim C, Minnerup J, Heindel W, Jeibmann A, Niederstadt T (2017) Ischemic stroke: histological thrombus composition and pre-interventional CT attenuation are associated with intervention time and rate of secondary embolism. Cerebrovasc Dis 44:344–350. https://doi.org/10.1159/000481578
Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, Nagatsuka K, Ishibashi-Ueda H, Kira JI, Toyoda K (2016) Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke 47:3035–3037. https://doi.org/10.1161/STROKEAHA.116.015228
Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, Yamasaki M, Kobayashi K, Sano T, Mori G, Yabana T, Naito Y, Shimizu S, Miya F (2018) Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra 8:39–49. https://doi.org/10.1159/000486042
Gupta AK, Nelson RC, Johnson GA, Paulson EK, Delong DM, Yoshizumi TT (2003) Optimization of eight-element multi-detector row helical CT technology for evaluation of the abdomen. Radiology 227:739–745. https://doi.org/10.1148/radiol.2273020591
Urikura A, Hara T, Ichikawa K, Nishimaru E, Hoshino T, Yoshida T, Nakaya Y (2016) Objective assessment of low-contrast computed tomography images with iterative reconstruction. Phys Med 32:992–998. https://doi.org/10.1016/j.ejmp.2016.07.003
van Ooijen PM, Ho KY, Dorgelo J, Oudkerk M (2003) Coronary artery imaging with multidetector CT: visualization issues. Radiographics 23:e16. https://doi.org/10.1148/rg.e16
Mair G, Boyd EV, Chappell FM, von Kummer R, Lindley RI, Sandercock P, Wardlaw JM, IST-3 Collaborative Group. Sensitivity and specificity of the hyperdense artery sign for arterial obstruction in acute ischemic stroke. Stroke. 2015;46:102–7. https://doi.org/10.1161/STROKEAHA.114.007036
Ahn SS, Kim EY (2012) Thrombus imaging in acute ischaemic stroke using thin-slice unenhanced CT: comparison of conventional sequential CT and helical CT. Eur Radiol 22:2392–2396. https://doi.org/10.1007/s00330-012-2518-y
Demchuk AM, Menon BK, Goyal M (2016) Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke 47:273–281. https://doi.org/10.1161/STROKEAHA.115.009171
Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR (2008) Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 191:409–415. https://doi.org/10.2214/AJR.07.3421
Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W, Stroke Thrombolysis Trialists' Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–35. https://doi.org/10.1016/S0140-6736(14)60584-5
Mazighi M, Chaudhry SA, Ribo M, Khatri P, Skoloudik D, Mokin M, Labreuche J, Meseguer E, Yeatts SD, Siddiqui AH, Broderick J, Molina CA, Qureshi AI, Amarenco P (2013) Impact of onset-to-reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation 127:1980–1985. https://doi.org/10.1161/CIRCULATIONAHA.112.000311
Tomsick T, Brott T, Barsan W, Broderick J, Haley EC, Spilker J, Khoury J (1996) Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. AJNR Am J Neuroradiol 17:79–85
Barber PA, Demchuk AM, Hudon ME, Pexman JHW, Hill MD, Buchan AM (2001) Hyperdense sylvian fissure MCA “dot” sign: A CT marker of acute ischemia. Stroke 32:84–88. https://doi.org/10.1161/01.STR.32.1.84
Akagi M, Nakamura Y, Higaki T, Narita K, Honda Y, Zhou J, Yu Z, Akino N, Awai K (2019) Deep learning reconstruction improves image quality of abdominal ultra-high-resolution CT. Eur Radiol 29:6163–6171. https://doi.org/10.1007/s00330-019-06170-3
Maegerlein C, Friedrich B, Berndt M, Lucia KE, Schirmer L, Poppert H, Zimmer C, Pelisek J, Boeckh-Behrens T, Kaesmacher J (2018) Impact of histological thrombus composition on preinterventional thrombus migration in patients with acute occlusions of the middle cerebral artery. Interv Neuroradiol 24:70–75. https://doi.org/10.1177/1591019917733733
Acknowledgements
We would like to thank Tomohiro Chiba (radiology technician at Kohnan Hospital) for providing an innovative idea. Takuya Saito, Yuichi Kawabata Akira Ito, and Fumihito Ichinohe contributed to the evaluation trial that preceded this paper. I also appreciate the cooperation of the members of the Radiology Department, representing radiology technicians at Ina Central Hospital.
Funding
This study was supported by a grant from the Daiwa Securities Health Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The Ina Central Hospital’s ethics committee approved the study protocol. This study is registered in the UMIN Registry, Japan (UMIN000040807).
Consent to participate
The patients or their relatives provided informed consent according to ethical regulations.
Consent for publication
All authors consent to publication. Patient consent and Ina Central Hospital ethics committee consent to publish were obtained.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobayashi, Y., Morizumi, T., Okumura, G. et al. Visualization of thrombus using iterative reconstruction and maximum intensity projection of thin-slice CT images. Neuroradiology 64, 2373–2379 (2022). https://doi.org/10.1007/s00234-022-02996-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-02996-9