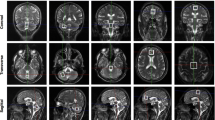
Abstract
Purpose
Inborn errors of neurotransmitters are rare monogenic diseases. In general, conventional neuroimaging is not useful for diagnosis. Nevertheless, advanced neuroimaging techniques could provide novel diagnosis and prognosis biomarkers. We aim to describe cerebral volumetric findings in a group of Spanish patients with neurotransmitter disorders.
Methods
Fifteen 3D T1-weighted brain images from the International Working Group on Neurotransmitter related Disorders Spanish cohort were assessed (eight with monoamine and seven with amino acid disorders). Volumes of cortical and subcortical brain structures were obtained for each patient and then compared with those of two healthy individuals matched by sex and age.
Results
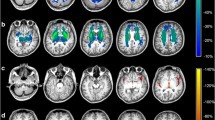
Regardless of the underlying disease, patients showed a smaller total cerebral tissue volume, which was apparently associated with clinical severity. A characteristic volumetric deficit pattern, including the right Heschl gyrus and the bilateral occipital gyrus, was identified. In severe cases, a distinctive pattern comprised the middle and posterior portions of the right cingulate, the left superior motor area and the cerebellum. In succinate semialdehyde dehydrogenase deficiency, volumetric affection seems to worsen over life.
Conclusion
Despite the heterogeneity and limited size of our cohort, we found novel and relevant data. Total volume deficit appears to be a marker of severity, regardless of the specific neurotransmitter disease and irrespective of the information obtained from conventional neuroimaging. Volumetric assessment of individual brain structures could provide a deeper knowledge about pathophysiology, disease severity and specific clinical traits.



Similar content being viewed by others
Abbreviations
- GABA:
-
Gamma-aminobutyric acid
- CSF:
-
Cerebrospinal fluid
- MRI:
-
Magnetic resonance imaging
- iNTD:
-
International Working Group on Neurotransmitter related Disorders
- AADCD:
-
Aromatic amino acid decarboxylase deficiency
- ADGTPCHD:
-
Autosomal dominant GTP cyclohydrolase deficiency
- ARGTPCHD:
-
Autosomal recessive GTP cyclohydrolase deficiency
- MAOA-BD:
-
Monoamine oxidase A and B deficiency
- NKH:
-
Non-ketotic hyperglycinemia
- PTPSD:
-
6-Pyruvoyl-tetrahydropterin synthase deficiency
- SSADHD:
-
Succinate-semialdehyde-dehydroxylase deficiency
- THD:
-
Tyrosine hydroxylase deficiency
References
Pearl PL, Wallis DD, Gibson KM (2004) Pediatric neurotransmitter diseases. Curr Neurol Neurosci Rep 4(2):147–152
Pearl PL (2013) Monoamine neurotransmitter deficiencies. Handb Clin Neurol 113:1819–1825
Ng J, Zhen J, Meyer E, Erreger K, Li Y, Kakaret N et al (2014) Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood. Brain 137(Pt 4):1107–1119
Brennenstuhl H, Jung-Klawitter S, Assmann B, Opladen T (2019) Inherited disorders of neurotransmitters: classification and practical approaches for diagnosis and treatment. Neuropediatrics 50(1):2–14
KuseyriHübschmann O, Horvath G, Cortès-Saladelafont E, Yıldız Y, Mastrangelo M, Pons R et al (2021) Insights into the expanding phenotypic spectrum of inherited disorders of biogenic amines. Nat Commun 20 12(1):5529
Kuseyri Hübschmann O, Mohr A, Friedman J, Manti F, Horvath G, Cortès-Saladelafont E et al (2021) Brain MR patterns in inherited disorders of monoamine neurotransmitters: an analysis of 70 patients. J Inherit Metab Dis 44(4):1070–1082
Ng J, Papandreou A, Heales SJ, Kurian MA (2015) Monoamine neurotransmitter disorders-clinical advances and future perspectives. Nat Rev Neurol 11(10):567–584
Van der Crabben SN, Verhoeven-Duif NM, Brilstra EH, Van Maldergem L, Coskun T, Rubio-Gozalbo E et al (2013) An update on serine deficiency disorders. J Inherit Metab Dis 36(4):613–619
Didiášová M, Banning A, Brennenstuhl H, Jung-Klawitter S, Cinquemani C, Opladen T et al (2020) Succinic semialdehyde dehydrogenase deficiency an update. Cells 19 9(2):477
Nonketotic Hyperglycinemia.2019 GeneReviews® [Internet] Seattle
Wei SH, Weng WC, Lee NC, Hwu WL, Lee WT (2011) Unusual spinal cord lesions in late-onset non-ketotic hyperglycinemia. J Child Neurol 26(7):900–903
Zubarioglu T, Kiykim E, Cansever MS, Aktuglu Zeybek C, Yalcinkaya C (2016) Neonatal nonketotic hyperglycinemia: diffusion-weighted magnetic resonance imaging and diagnostic clues. Acta Neurol Belg 116(4):671–673
Lee W, Weng W, Peng S, Tzen K (2009) Neuroimaging findings in children with paediatric neurotransmitter diseases. J Inherit Metab Dis 32(3):361–370
Yamakawa Y, Nakazawa T, Ishida A, Saito N, Komatsu M, Matsubara T et al (2012) A boy with a severe phenotype of succinic semialdehyde dehydrogenase deficiency. Brain Dev 34(2):107–112
Damseh N, Simonin A, Jalas C, Picoraro JA, Shaag A, Cho MT et al (2015) Mutations in SLC1A4, encoding the brain serine transporter, are associated with developmental delay, microcephaly and hypomyelination. J Med Genet 52(8):541–547
de Koning TJ, Jaeken J, Pineda M, Van Maldergem L, Poll-The BT, van der Knaap MS (2000) Hypomyelination and reversible white matter attenuation in 3-phosphoglycerate dehydrogenase deficiency. Neuropediatrics 31(6):287–292
Bjoraker KJ, Swanson MA, Coughlin CR, Christodoulou J, Tan ES, Fergeson M et al (2016) Neurodevelopmental outcome and treatment efficacy of benzoate and dextromethorphan in siblings with attenuated non-ketotic hyperglycinemia. J Pediatr 170:234–239
Avants B, Epstein C, Grossman M, Gee J (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41
Ye J, Yang Y, Yu W, Zou H, Jiang J, Yang R et al (2013) Demographics, diagnosis and treatment of 256 patients with tetrahydrobiopterin deficiency in mainland China: results of a retrospective, multicentre study. J Inherit Metab Dis 36(5):893–901
Mohammad SA, Abdelkhalek HS (2017) Nonketotic hyperglycinemia: spectrum of imaging findings with emphasis on diffusion weighted imaging. Neuroradiology 59:1155–1163
Stence NV, Fenton LZ, Levek C, Tong S, Coughlin CR, Hennermann JB et al (2019) Brain imaging in classic nonketotic hyperglycinemia: quantitative analysis and relation to phenotype. J Inherit Metab Dis 42(3):438–450
Lakhani R, Vogel KR, Till A, Liu J, Burnett SF, Gibson KM, Subramani S (2014) Defects in GABA metabolism affect selective autophagy pathways and are alleviated by mTOR inhibition. EMBO Mol Med 6(4):551–66. https://doi.org/10.1002/emmm.201303356
Wassenberg T, Molero-Luis M, Jeltsch K, Hoffmann GF, Assmann B, Blau N et al (2017) Consensus guideline for the diagnosis and treatment of aromatic l amino acid decarboxylase (AADC) deficiency. Orphanet J Rare Dis 18(12):1–12
Lee WT, Lin JH, Weng WC, Peng SF (2016) Microstructural changes of brain in patients with aromatic L-amino acid decarboxylase deficiency. Hum Brain Mapp 38(3):1532–1540
Xing L, Huttner WB (2020) Neurotransmitters as modulators of neural progenitor cell proliferation during mammalian neocortex development. Front Cell Dev Biol 8:391
Cameron HA, Hazel TG, Mckay RD (1998) Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol 36:287–306
Salazar P, Velasco-Velazquez MA, Velasco I (2008) GABA effects during neuronal differentiation of stem cells. Neurochem Res 33:1546–1557
Van Horn MR, Sild M, Ruthazer ES (2013) D-serine as a gliotransmitter and its roles in brain development and disease. Front Cell Neurosci 23(7):39
Zhang Y, Bhavnani BR (2006) Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci 7:49
Tabatabaie L, Klomp LW, Berger R, de Koning TJ (2010) l-Serine synthesis in the central nervous system: a review on serine deficiency disorders. Mol Genet Metab 99(3):256–262
Acknowledgements
We thank the patients and their parents for their support and participation in this study.
Funding
This work was supported by the FIS P118/00111 “Instituto de Salud Carlos III (ISCIII)” and “Fondo Europeo de desarrollo regional (FEDER)”.
Author information
Authors and Affiliations
Contributions
Chiara Alfonsi: conceptualization; data curation; resources; formal analysis; investigation; methodology; writing—original draft.
Christian Stephan-Otto: conceptualization; formal analysis; investigation; methodology; resources; software; validation; writing—original draft.
Elisenda Cortès-Saladelafont: conceptualization; data curation; writing—original draft.
Inés Podzamczer-Valls: data curation.
Natalia Juliá Palacios, Nuria Gutiérrez Cruz, María Rosario Domingo Jiménez, Salvador Ibáñez Micó, Miguel Tomás Vila, Ramón Velázquez Fragua, Teresa Gómez, Oscar Alcoverro Fortuny, Inmaculada García Jiménez, Eduardo López Laso, Ana Roche Martínez: data curation; resources; writing—review and editing.
Kathrin Jeltsch, Oya Kuseyri Hübschmann, Thomas Opladen: writing—review and editing.
Muchart Jordi López: data curation; software; resources; writing—review and editing.
Àngels Garcia-Cazorla: conceptualization; funding acquisition; investigation; project administration; resources; supervision; validation; visualisation; writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the local ethics committee (ID number: PIC-131–18). All the procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Consent to participate
Oral and written informed consent was obtained from all subjects or their parents/legal guardians regarding publishing their data. Images from control subjects were obtained from a local anonymized MRI database, for which the patients or their parents/legal guardians gave informed consent.
Conflict of interest
Dr. Cortès-Saladelafont reports personal fees from Takeda, outside the submitted work. Dr. Kuseyri Hübschmann reports personal fees from PTC Therapeutics GT, outside the submitted work. Dr. López-Laso has received honoraria as an invited speaker and has also received consultation fees from PTC Therapeutics, outside the submitted work. Dr. Ibáñez-Micó reports grants and personal fees from PTC Therapeutics, during the conduct of the study. Dr. Alfonsi, Dr. Stephan-Otto, Dr. Juliá Palacios, Dr. Podzamczer-Valls, Dr. Nuria Gutiérrez, Dr. Kathrin Jeltsch, Dr. Velázquez Fragua, Dr. Alcoverro-Fortuny, Dr. Teresa Gómez, Dr. Roche Martínez and Dr. Muchart López have nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alfonsi, C., Stephan-Otto, C., Cortès-Saladelafont, E. et al. Volumetric study of brain MRI in a cohort of patients with neurotransmitter disorders. Neuroradiology 64, 2179–2190 (2022). https://doi.org/10.1007/s00234-022-02989-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-02989-8