Abstract
Purpose
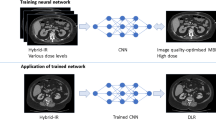
Deep learning-based reconstruction (DLR) has been developed to reduce image noise and increase the signal-to-noise ratio (SNR). We aimed to evaluate the efficacy of DLR for high spatial resolution (HR)-MR cisternography.
Methods
This retrospective study included 35 patients who underwent HR-MR cisternography. The images were reconstructed with or without DLR. The SNRs of the CSF and pons, contrast of the CSF and pons, and sharpness of the normal-side trigeminal nerve using full width at half maximum (FWHM) were compared between the two image types. Noise quality, sharpness, artifacts, and overall image quality of these two types of images were qualitatively scored.
Results
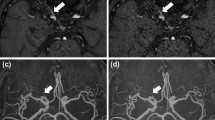
The SNRs of the CSF and pons were significantly higher with DLR than without DLR (CSF 21.81 ± 7.60 vs. 15.33 ± 4.03, p < 0.001; pons 5.96 ± 1.38 vs. 3.99 ± 0.48, p < 0.001). There were no significant differences in the contrast of the CSF and pons (p = 0.225) and sharpness of the normal-side trigeminal nerve using FWHM (p = 0.185) without and with DLR, respectively. Noise quality and the overall image quality were significantly higher with DLR than without DLR (noise quality 3.95 ± 0.19 vs. 2.53 ± 0.44, p < 0.001; overall image quality 3.97 ± 0.17 vs. 2.97 ± 0.12, p < 0.001). There were no significant differences in sharpness (p = 0.371) and artifacts (p = 1) without and with DLR.
Conclusion
DLR can improve the image quality of HR-MR cisternography by reducing image noise without sacrificing contrast or sharpness.






Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- DCT:
-
Discrete cosine transform
- DLR:
-
Deep learning-based reconstruction
- FASE:
-
Fast asymmetric spin-echo
- FWHM:
-
Full width at half maximum
- HR:
-
High-spatial resolution
- SNR:
-
Signal-to-noise ratio
- T1WI:
-
T1-weighted image
- T2WI:
-
T2-weighted image
- CNR:
-
Contrast-to-noise ratio
References
Naganawa S, Koshikawa T, Fukatsu H, Ishigaki T, Fukuta T (2001) MR cisternography of the cerebellopontine angle: comparison of three-dimensional fast asymmetrical spin-echo and three-dimensional constructive interference in the steady-state sequences. AJNR Am J Neuroradiol 22:1179–1185
Liu P, Saida Y, Yoshioka H, Itai Y (2003) MR imaging of epidermoids at the cerebellopontine angle. Magn Reson Med Sci 2:109–115
Masuda Y, Yamamoto T, Akutsu H, Shiigai M, Masumoto T, Ishikawa E, Matsuda M, Matsumura A (2015) Usefulness of subtraction of 3D T2WI-DRIVE from contrast-enhanced 3D T1WI: preoperative evaluations of the neurovascular anatomy of patients with neurovascular compression syndrome. AJNR Am J Neuroradiol 36:317–322
Nowe V, De Ridder D, Van de Heyning PH, Wang XL, Gielen J, Van Goethem J, Ozsarlak O, De Schepper AM, Parizel PM (2004) Does the location of a vascular loop in the cerebellopontine angle explain pulsatile and non-pulsatile tinnitus? Eur Radiol 14:2282–2289
Ryu H, Tanaka T, Yamamoto S, Uemura K, Takehara Y, Isoda H (1999) Magnetic resonance cisternography used to determine precise topography of the facial nerve and three components of the eighth cranial nerve in the internal auditory canal and cerebellopontine cistern. J Neurosurg 90:624–634
Hentschel MA, Kunst HPM, Rovers MM, Steens SCA (2018) Diagnostic accuracy of high-resolution T2-weighted MRI vs contrast-enhanced T1-weighted MRI to screen for cerebellopontine angle lesions in symptomatic patients. Clin Otolaryngol 43:805–811
Gamaleldin OA, Donia MM, Elsebaie NA, Abdelkhalek Abdelrazek A, Rayan T, Khalifa MH (2020) Role of fused three-dimensional time-of-flight magnetic resonance angiography and 3-dimensional T2-weighted imaging sequences in neurovascular compression. World Neurosurg 133:e180–e186
Kanoto M, Toyoguchi Y, Hosoya T, Oda A, Sugai Y (2013) Visualization of the trochlear nerve in the cistern with use of high-resolution turbo spin-echo multisection motion-sensitized driven equilibrium. AJNR Am J Neuroradiol 34:1434–1437
Aja-Fernandez S, Vegas-Sanchez-Ferrero G, Tristan-Vega A (2014) Noise estimation in parallel MRI: GRAPPA and SENSE. Magn Reson Imaging 32:281–290
Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P (1999) SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42:952–962
Monch S, Sollmann N, Hock A, Zimmer C, Kirschke JS, Hedderich DM (2019) Magnetic resonance imaging of the brain using compressed sensing - quality assessment in daily clinical routine. Clin Neuroradiol 30:279–286. https://doi.org/10.1007/s00062-019-00789-x
Zhang T, Chowdhury S, Lustig M, Barth RA, Alley MT, Grafendorfer T, Calderon PD, Robb FJ, Pauly JM, Vasanawala SS (2014) Clinical performance of contrast enhanced abdominal pediatric MRI with fast combined parallel imaging compressed sensing reconstruction. J Magn Reson Imaging 40:13–25
Sharma SD, Fong CL, Tzung BS, Law M, Nayak KS (2013) Clinical image quality assessment of accelerated magnetic resonance neuroimaging using compressed sensing. Investig Radiol 48:638–645
Chilamkurthy S, Ghosh R, Tanamala S, Biviji M, Campeau NG, Venugopal VK, Mahajan V, Rao P, Warier P (2018) Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet 392:2388–2396
Ueda D, Yamamoto A, Nishimori M, Shimono T, Doishita S, Shimazaki A, Katayama Y, Fukumoto S, Choppin A, Shimahara Y, Miki Y (2019) Deep learning for MR angiography: automated detection of cerebral aneurysms. Radiology 290:187–194
Perkuhn M, Stavrinou P, Thiele F, Shakirin G, Mohan M, Garmpis D, Kabbasch C, Borggrefe J (2018) Clinical evaluation of a multiparametric deep learning model for glioblastoma segmentation using heterogeneous magnetic resonance imaging data from clinical routine. Investig Radiol 53:647–654
Lin L, Dou Q, Jin YM, Zhou GQ, Tang YQ, Chen WL, Su BA, Liu F, Tao CJ, Jiang N, Li JY, Tang LL, Xie CM, Huang SM, Ma J, Heng PA, Wee JTS, Chua MLK, Chen H, Sun Y (2019) Deep learning for automated contouring of primary tumor volumes by MRI for nasopharyngeal carcinoma. Radiology 291:677–686
Dunnmon JA, Yi D, Langlotz CP, Ré C, Rubin DL, Lungren MP (2019) Assessment of convolutional neural networks for automated classification of chest radiographs. Radiology 290:537–544
Wang H, Zhang J, Bao S, Liu J, Hou F, Huang Y, Chen H, Duan S, Hao D, Liu J (2020) Preoperative MRI-based radiomic machine-learning nomogram may accurately distinguish between benign and malignant soft-tissue lesions: a two-center study. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27111
Jiang D, Dou W, Vosters L, Xu X, Sun Y, Tan T (2018) Denoising of 3D magnetic resonance images with multi-channel residual learning of convolutional neural network. Jpn J Radiol 36:566–574
Zhang K, Zuo W, Chen Y, Meng D, Zhang L (2017) Beyond a Gaussian denoiser: residual learning of deep CNN for image denoising. IEEE Trans Image Process 26:3142–3155
Kidoh M, Shinoda K, Kitajima M, Isogawa K, Nambu M, Uetani H, Morita K, Nakaura T, Tateishi M, Yamashita Y, Yamashita Y (2019) Deep learning based noise reduction for brain MR imaging: tests on phantoms and healthy volunteers. Magn Reson Med Sci 19:195–206. https://doi.org/10.2463/mrms.mp.2019-0018
Isogawa K, Ida T, Shiodera T, Takeguchi T (2018) Deep shrinkage convolutional neural network for adaptive noise reduction. IEEE Signal Process Lett 25:224–228
Erturk MA, Bottomley PA, El-Sharkawy AM (2013) Denoising MRI using spectral subtraction. IEEE Trans Biomed Eng 60:1556–1562
Sijbers J, den Dekker AJ, Van der Linden A, Verhoye TM, Van Dyck D (1999) Adaptive anisotropic noise filtering for magnitude MR data. Magn Reson Imaging 17:1533–1539
Donoho DL (1995) De-noising by soft-thresholding. IEEE Trans Inf Theory 41:613–627
Laine AF (2000) Wavelets in temporal and spatial processing of biomedical images. Annu Rev Biomed Eng 2:511–550
Yang X, Fei B (2011) A wavelet multiscale denoising algorithm for magnetic resonance (MR) images. Meas Sci Technol 22:25803
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Hiroyuki Uetani, Tadashi Hamasaki, Machiko Tateishi, Kosuke Morita, Akira Sasao, and Seitaro Oda. The first draft of the manuscript was written by Hiroyuki Uetani, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Conceptualization: Hiroyuki Uetani and Takeshi Nakaura
Data collection: Hiroyuki Uetani, Tadashi Hamasaki, Machiko Tateishi, Kosuke Morita, Akira Sasao, and Seitaro Oda
Methodology: Hiroyuki Uetani, Takeshi Nakaura, Mika Kitajima, and Yuichi Yamashita
Formal analysis and investigation: Hiroyuki Uetani, Takeshi Nakaura, Mika Kitajima, and Machiko Tateishi
Writing—original draft preparation: Hiroyuki Uetani
Writing—review and editing: Takeshi Nakaura, Mika Kitajima, Osamu Ikeda, and Yasuyuki Yamashita
Supervision: Takeshi Nakaura, Mika Kitajima, Osamu Ikeda, and Yasuyuki Yamashita
Corresponding author
Ethics declarations
Conflict of interest
Yuichi Yamashita is an employee of Canon Medical Systems. The other authors declare that they have no conflicts of interest.
Ethical approval
All procedures followed the clinical study guidelines of the ethics committee of Kumamoto university hospital (Kumamoto, Japan), and they were approved by our institutional review board.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uetani, H., Nakaura, T., Kitajima, M. et al. A preliminary study of deep learning-based reconstruction specialized for denoising in high-frequency domain: usefulness in high-resolution three-dimensional magnetic resonance cisternography of the cerebellopontine angle. Neuroradiology 63, 63–71 (2021). https://doi.org/10.1007/s00234-020-02513-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02513-w