Abstract
Purpose
The clinical presentation of idiopathic normal pressure hydrocephalus (iNPH) may overlap with progressive supranuclear palsy (PSP). The Magnetic Resonance Parkinsonism Index (MRPI), MRPI 2.0, and the interpeduncular angle (IPA) have been investigated to differentiate PSP from healthy controls (HC) and other parkinsonisms. We aimed to assess equivalences and differences in MRPI, MRPI 2.0, and IPA in iNPH, PSP, and HC groups.
Methods
We retrospectively recruited 99 subjects (30 iNPH, 32 PSP, 37 HC) from two institutions. MRI exams, acquired on either 1.5 T or 3 T scanners, included 3D T1-weighted images to measure MRPI, MRPI 2.0, and IPA. Inter- and intra-rater reliability was investigated with the intra-class correlation coefficient (ICC), and the two one-sided t tests (TOST) procedure was used to assess these markers in iNPH, PSP, and HC.
Results
For all the three measures, intra-rater and inter-rater ICC were excellent (range = 0.91–0.93).
In the comparison of iNPH and PSP with HC, differences for MRPI and MRPI 2.0 (p < 0.01 in all cases) and no equivalence (p = 1.00 in all cases) were found at TOST. iNPH and PSP MRPI showed no difference (p = 0.06) and no equivalence (p = 0.08). MRPI 2.0 was not equivalent (p = 0.06) and not different (p = 0.09) in the same two populations. PSP and HC IPA proved equivalent (p < 0.01) while iNPH IPA was different (p < 0.01) and not equivalent (p = 0.96 and 0.82) from both PSP and HC.
Conclusion
MRPI and MRPI 2.0 significantly overlap in iNPH and PSP, with risk of misdiagnosis, and for this reason may not be helpful in the differential diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic normal pressure hydrocephalus (iNPH) is a potentially treatable syndrome characterized by a variable combination of impaired gait, cognition, and urinary dysfunction (urgency and incontinence) [1]. iNPH can be diagnosed by medical history, neurologic examination, and brain imaging with CT or MRI. An early diagnosis is essential to achieve an optimal treatment outcome and to avoid irreversible impairments. However, the differential diagnosis can be challenging, because the clinical spectrum of iNPH overlaps with that of other entities, especially atypical parkinsonisms. In particular, progressive supranuclear palsy (PSP) shares with iNPH some of the cardinal clinical features, i.e., gait dysfunction, postural instability with retropulsion, and cognitive impairment [2].
Clinically, PSP could be distinguished from iNPH based on other specific features, in particular the typical ocular motor dysfunction, characterized by supranuclear gaze palsy or slowing of vertical saccades. Nevertheless, the ocular motor dysfunction can be missing in the early stages of PSP, especially in non-Richardson’s phenotypes [2]. On the other side, the urinary dysfunction is a classical feature of iNPH, but can be a non-specific and frequent finding in a population older than 60 years [3].
To improve diagnostic accuracy of PSP and iNPH, various neuroimaging indices have been proposed in the last few years. Magnetic Resonance Parkinsonism Index (MRPI) has been introduced by Quattrone et al. in 2008 [4] to recognize patients with PSP, and has therefore proved useful in helping clinicians to consolidate the diagnosis based on clinical features. As an extension of this metric, the MRPI 2.0, including the measurement of the third ventricle width and of the frontal horn distance, has been more recently introduced, which showed a superior accuracy, as compared with MRPI, in differentiating PSP patients from those with early stage Parkinson’s disease (PD) [5]. Additionally, interpeduncular angle (IPA) has been proposed to differentiate PSP patients from other parkinsonisms, with discordant results [6, 7]. On the other hand, the callosal angle and Evans index have proven effective in helping the radiologist differentiate patients with iNPH [8].
These indices proved to be useful in distinguishing PSP and iNPH from healthy controls and from other neurodegenerative diseases, but less useful to distinguish PSP and iNPH between each other [9].
Given the clinical but also radiological similarities between these two diseases, the aim of our study was to evaluate different MRI measurements (MRPI, MRPI 2.0, IPA) between PSP, iNPH, and healthy controls (HCs).
Material and methods
Participants
The present work has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
We retrospectively analyzed the digital records at two different institutions to find iNPH and PSP patients who underwent MRI exams between January 2014 and December 2018. In all cases, only retrospective, anonymized information was used for the study; therefore, individual written informed consent was waived by the local IRBs (Comitato etico Università Federico II, Naples, Italy; Comitato Etico Lazio 2, Rome, Italy). Their inclusion was based on a diagnosis of “probable” disease in accordance with international guidelines [2, 10,11,12], made by a movement disorder specialist. Exclusion criteria were unavailability of a 3D isotropic T1-weighted (T1w) sequence, artifacts on the images used for the analysis, or the presence of significant neurological comorbidities. We exclusively selected the first MRI exam undergone by each patient. In this manner, we assessed the usefulness of the MRPI indices and IPA at the time of initial diagnosis, the ideal clinical application of these biomarkers. Then, a group of HC previously enrolled in other studies at the same institutions and whose exams also included 3D isotropic T1w images, was selected for the analysis.
MR data acquisition and analysis
MR examinations were performed on three different scanners (1.5 Tesla Gyroscan Intera, Philips, Eindhoven, The Netherlands; Magnetom Espree, Siemens Healthineers, Erlangen, Germany; 3 Tesla Magnetom Trio, Siemens Healthineers, Erlangen, Germany). A complete list of all acquisition details and parameters is available in the supplementary materials.
Using 3D T1w isotropic images, MRPI and MRPI 2.0 were calculated as previously described [4, 5]. In particular, the midbrain and pons areas, divided by a line passing through the superior pontine notch and the inferior edge of the quadrigeminal plate, were measured on midsagittal T1w MR images. Middle cerebellar peduncles (MCP) were identified on parasagittal views, while superior cerebellar peduncles (SCP) were measured on oblique coronal MR image tangent to the floor of the fourth ventricle. The 3rd ventricle width was measured on an axial slice generated at the level of both the anterior and posterior commissures by averaging three different measurements of the maximum linear distance between the lateral borders. The frontal horn distance was evaluated on the axial view showing their maximal dilatation, and the largest left-to-right width was measured. MRPI was calculated by multiplying the midsagittal area of the pons/midsagittal area of the midbrain ratio by the MCP width/SCP width ratio. MRPI 2.0 values were obtained by multiplying the MRPI value by the 3rd ventricle width/frontal horn width ratio.
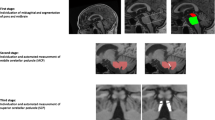
Finally, the IPA was also calculated for all subjects, defined as the angle formed by the posterior half of the cerebral peduncles at the level of the mammillary bodies or immediately below [13]. Two examples of the obtained measures are available in Figs. 1 and 2 for an iNPH and a PSP patient, respectively.
Sagittal (a–c) and axial (d–e) T1-weighted volumetric MR images of an iNPH patient showing sections on which MRPI and MRPI 2.0 measurements were performed. Midbrain and pons areas (a), middle (b), and superior (c) cerebellar peduncles thickness, frontal horn distance (d), and 3rd ventricle width (e) are depicted. Axial T1-weighted section on which interpeduncular angle was measured (f) is also shown
Image analysis was performed independently by two raters (both with 8 years of experience).
Statistical analysis
The obtained values were analyzed via equivalence testing following the two one-sided t tests (TOST) procedure corrected for multiple comparisons [14, 15]. This test takes into consideration the difference between “equivalent” and “not different.” The first implies confidence in stating there is no practical difference between the groups being compared, while the second that there was not sufficient evidence to determine they were different [16]. In TOST, the first one-sided test compares the mean with the lower equivalence bound and the second with the upper one, employing the larger p value to determine the result’s significance [14].
As populations were of different sample sizes, variance was not assumed as equal and Welch’s t test was employed. Equivalence bounds to use in the TOST were obtained through a preliminary power analysis with a desired power of 80%, an alpha value of 0.025, the sample size of the smaller group available in each comparison, and a pooled standard deviation obtained with Cohen’s formula. Differences between gender distribution were assessed by Fisher’s exact test, while continuous variables were compared using unpaired t tests.
The intra- and inter-rater reproducibility was calculated using the intra-class correlation coefficient (ICC). To assess the intra-rater reliability, one of the two raters performed a second evaluation after a 4-week washout period.
In detail, inter-rater reproducibility was calculated using a single rater, absolute agreement, two-way random effects model while the intra-rater with a single rater, consistency, two-way mixed effects one. The results were interpreted following the scale suggested by Koo and Li: poor (< 0.5), moderate (0.5–0.75), good (0.75–0.9), and excellent (> 0.9) [17].
All analyses were conducted using the R statistical software (R for Unix/Linux, version 3.4.4, the R Foundation for Statistical Computing, 2014) [18]; a p value < 0.05 was considered statistically significant with corrections for multiple comparisons when necessary.
Results
Demographic data and MRI indices for the three groups are reported in Table 1. Mean age was 71.17 years (± 7.52) for iNPH, 72.19 (± 5.67) for PSP, and 69.09 (± 4.66) for control populations. Age distribution was normal for all groups (p = 0.35–0.50) without significant differences at ANOVA (p = 0.08). Similarly, no significant differences were found in terms of gender distribution (p = 0.10).
Both intra-rater and inter-rater agreements proved to be excellent (ICC = 0.93 for MRPI, 0.92 for MRPI 2.0 and 0.92 for IPA; ICC = 0.92 for MRPI; 0.91 for MRPI 2.0 and 0.92 for IPA, respectively). The data for ICC analysis is available in the supplementary materials together with Bland-Altman plots for MRPI, MRPI 2.0, and IPA intra- and inter-rater agreement.
Figure 3 shows the distribution of MRPI, MRPI 2.0, and IPA. In detail, iNPH patients had an average MRPI of 15.23 (± 3.23), MRPI 2.0 of 4.57 (± 1.37), and 83.50° IPA (± 6.76°). For the PSP group, these were respectively 17.01 (± 4.08), 3.99 (± 1.30), and 75.38° (± 5.72°). Finally, controls had 8.63 (± 1.38), 1.40 (± 0.48), and 75.53° (± 8.07°) averages for MRPI, MRPI 2.0, and IPA.
Results of the comparisons performed with the corrected TOST procedure are illustrated in Table 2 and Figs. 4, 5, and 6. Briefly, no significant differences (p = 0.06) and no significant equivalence (p = 0.08) were found in MRPI score between iNPH and PSP patients. Similarly, MRPI 2.0 was non-equivalent (p = 0.06) and not different (p = 0.09) between these two patient groups. On the other hand, the comparison between iNPH patients and HC, as well as between PSP patients and HC, showed significant differences both for MRPI and MRPI 2.0 (p < 0.01 in all cases) and no equivalence (p = 1.00 in all cases). When the IPA measurements were evaluated, this metric proved to be equivalent between PSP patients and HC (p < 0.01), and not different (p = 0.93), while it was significantly higher in iNPH patients compared with both PSP and control groups (p < 0.01 in both cases), being not equivalent (p = 0.96 and 0.82, respectively).
Discussion
In this study we evaluated the possible differences between iNPH, PSP, and HC in terms of different MRI metrics used in clinical practice. We found that both MRPI and MRPI 2.0 scores were not significantly different between iNPH and PSP patients, thus allowing us to suggest that these metrics could lead to a wrong neuroradiological evaluation in clinical practice.
iNPH represents the most common form of hydrocephalus in adults with a probable iNPH estimated prevalence of 0.2% in patients aged 70–79 years and 5.9% in patients aged 80 years and older, with no difference between men and women [19]. To date, the only effective treatment for iNPH is the shunt surgery [20, 21]. Nevertheless, only a part of iNPH patients achieve a significant clinical improvement after treatment, with different tests used to identify patients who are likely to respond to shunt surgery, including the tap test or CSF infusion testing [22]. It should be noted that from a clinical standpoint, different extra-pyramidal syndromes can overlap with findings found in iNPH. Among these, the most prominent differential diagnosis stands with PSP. Indeed, pure akinesia with gait freezing, accompanied by unsteadiness and falls, may be present in both groups of patients [23]. For this reason, the presence of normal pressure or obstructive hydrocephalus on imaging represents a mandatory exclusion criterion for a proper PSP diagnosis [2].
From a radiological standpoint, brain MRI changes in iNPH patients include ventriculomegaly, acute callosal angle, disproportionate changes in subarachnoid spaces with dilated Sylvian fissures, and narrow sulci and subarachnoid spaces at the vertex and medial/parafalcine region, defined as disproportionately enlarged subarachnoid-space hydrocephalus (DESH) [24, 25]. On the other hand, PSP is a progressive neurological disorder radiologically characterized by presence of midbrain atrophy and, to a lesser extent, supratentorial structures with ex vacuo dilation of the ventricle-cisternal system [26]. In detail, in a recent research Pyatigorskaya and colleagues performed a precise in vivo staging of neurodegeneration in PSP using quantitative multimodal MRI at 3 and 7 Tesla showing extensive volume decreases and diffusion changes in the midbrain, substantia nigra, subthalamic nucleus, globus pallidus, basal forebrain, locus coeruleus, pedunculopontine nucleus, and dentate nucleus, overlapping degrees of impairment in histological analyses [27].
In the last years, MRPI showed excellent performance in recognizing PSP patients, and in differentiating them from patients with PD, and for this reason, its clinical usage in auxiliary diagnosis of PSP is strongly recommended [28].
Our results show no difference in MRPI between PSP and iNPH patients, leading to several considerations. Firstly, the increased size of the third ventricle in iNPH patients produces a widening of the cerebral peduncles, as demonstrated by the higher IPA values compared with those found in the HC. Given that the mesencephalic measurements are performed on the midsagittal slice, this may lead to an underestimation of the mesencephalic volume in iNPH patients. Even the inclusion of ventricular dilation markers in MRPI 2.0, compared with MRPI, does not solve this overlap in imaging findings, as shown in our results. Volume-based indices might be able to effectively quantify mesencephalic atrophy or superior cerebellar peduncle volume, even though their use in daily clinical practice is still limited [29]. Furthermore, the presence of a tortuous posterior circulation in some older iNPH patients leading to an upper displacement of the third ventricle floor by posterior cerebral arteries may contribute to alter the midsagittal mesencephalic morphology. This might produce flattening or concave outline to the superior aspect of the midbrain, which should be upwardly convex, possibly mimicking the hummingbird sign of PSP patients (Fig. 7).
In regard to the statistical power of our findings, it should be noted that the equivalence bounds were calculated as to ensure an 80% power. We wish to highlight that the resulting values were above the suggested cut-off proposed by Quattrone and colleagues both for MRPI and MRPI 2.0 in the differential diagnosis between PSP-P and HC (respectively 11.34 and 2.18) [5]. This supports the overlap in said scores between iNPH and PSP-P patients; both clearly increased compared with healthy subjects. In line with previous studies, we found an excellent reproducibility of all MRI metrics in our population [4, 5, 13], further corroborating the use of these measures in clinical practice.
A recent publication by Constantinides and colleagues investigated quantitative and qualitative MRI signs including MRPI in PSP, iNPH, and HC groups [30]. They report a difference in terms of MRPI between PSP and iNPH patients with a p value of 0.049. In our study, the same parameter showed no differences between these groups with a p value of 0.06. This could be explained by the different sizes of their iNPH group (n = 17 vs 30). Nonetheless, their conclusion further supports the imaging overlap between PSP and iNPH as none of the markers analyzed proved reliable in their differential diagnosis. In this setting, the differences we found in IPA between iNPH and both PSP and HC are of particular interest. This finding is further reinforced by the unequivocal equivalence of IPA values in the PSP and HC groups. For this reason, the IPA value might be a useful tool in the radiological evaluation of these patients, in addition to other already established measurements such as the callosal angle.
This study has some limitations which have to be pointed out. First of all, iNPH and PSP diagnoses were made by a movement disorder specialist with a “probable” level of diagnostic certainty, and not pathologically confirmed. This may have partially affected the results, since some patients with antemortem diagnosis of iNPH have been noted to have coexisting neurodegenerative pathologies including PSP on neuropathology [23]. Disease duration at the moment of MRI evaluation has not been taken into account. We are aware that disease duration and stage could impact the imaging presentation of these patients, while it has been reported that MRPI can detect abnormalities in very early stages of disease [31,32,33]; to address this issue the patient’s first MR study since clinical onset was considered. While the power analysis supports the validity of our findings, further studies on larger populations are obviously mandatory, to confirm our results. In particular, we think that a specific prospective investigation about the role of IPA to differentiate between iNPH and PSP patients is strongly warranted, given the findings of this study. Furthermore, the different Movement Disorder Society PSP subtypes were not considered in the present study, although it should be noted that in a recent study Picillo and colleagues showed that MRPI and MRPI 2.0 values are not significantly different among several PSP subtypes [34].
Conclusion
Our study showed that MRPI and MRPI 2.0 scores may not be helpful in the differential diagnosis between PSP and iNPH, given the overlap of these metrics. On the other hand, IPA was generally higher in iNPH than in PSP patients and in HC; therefore, it demonstrated a useful additional marker to differentiate this potentially treatable condition.
Abbreviations
- iNPH:
-
Idiopathic normal pressure hydrocephalus
- PSP:
-
Progressive supranuclear palsy
- MRPI:
-
Magnetic Resonance Parkinsonism Index
- PD:
-
Parkinson disease
- IPA:
-
Interpeduncular angle
- HC:
-
Healthy controls
- MCP:
-
Middle cerebellar peduncles
- SCP:
-
Superior cerebellar peduncles
- TOST:
-
Two one-sided t tests
- ICC:
-
Intra-class correlation coefficient
References
Williams MA, Malm J (2016) Diagnosis and treatment of idiopathic normal pressure hydrocephalus. Contin Lifelong Learn Neurol 22:579–599. https://doi.org/10.1212/CON.0000000000000305
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, for the Movement Disorder Society-endorsed PSP Study Group (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864. https://doi.org/10.1002/mds.26987
Skalický P, Mládek A, Vlasák A, de Lacy P, Beneš V, Bradáč O (2019) Normal pressure hydrocephalus—an overview of pathophysiological mechanisms and diagnostic procedures. Neurosurg Rev. https://doi.org/10.1007/s10143-019-01201-5
Quattrone A, Nicoletti G, Aguglia U (2008) MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple purpose : methods : results : conclusion. Radiology 246:214–221
Quattrone A, Morelli M, Nigro S, Quattrone A, Vescio B, Arabia G, Nicoletti G, Nisticò R, Salsone M, Novellino F, Barbagallo G, le Piane E, Pugliese P, Bosco D, Vaccaro MG, Chiriaco C, Sabatini U, Vescio V, Stanà C, Rocca F, Gullà D, Caracciolo M (2018) A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Park Relat Disord 54:3–8. https://doi.org/10.1016/j.parkreldis.2018.07.016
Tipton PW, Konno T, Broderick DF, Dickson DW, Wszolek ZK (2016) Cerebral peduncle angle: unreliable in differentiating progressive supranuclear palsy from other neurodegenerative diseases. Parkinsonism Relat Disord 32:31–35. https://doi.org/10.1016/j.parkreldis.2016.08.009
Eraslan C, Acarer A, Guneyli S, Akyuz E, Aydin E, Colakoglu Z, Kitis O, Calli MC (2019) MRI evaluation of progressive supranuclear palsy: differentiation from Parkinson’s disease and multiple system atrophy. Neurol Res 41:110–117. https://doi.org/10.1080/01616412.2018.1541115
Miskin N, Patel H, Franceschi AM, Ades-Aron B, le A, Damadian BE, Stanton C, Serulle Y, Golomb J, Gonen O, Rusinek H, George AE, For the Alzheimer’s Disease Neuroimaging Initiative (2017) Diagnosis of normal-pressure hydrocephalus: use of traditional measures in the era of volumetric MR imaging. Radiology 285:197–205. https://doi.org/10.1148/radiol.2017161216
Ohara M, Hattori T, Yokota T (2020) Progressive supranuclear palsy often develops idiopathic normal pressure hydrocephalus-like MRI features. Eur J Neurol ene.14322. https://doi.org/10.1111/ene.14322
Relkin N, Marmarou A, Klinge P et al (2005) Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 57:S2-4–S2-16. https://doi.org/10.1227/01.NEU.0000168185.29659.C5
Marmarou A, Bergsneider M, Klinge P et al (2005) The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 57:S2-17–S2-28. https://doi.org/10.1227/01.NEU.0000168184.01002.60
Mori E, Ishikawa M, Kato T et al (2012) Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo) 52:775–809. https://doi.org/10.2176/nmc.52.775
Wang DJ, Pandey SK, Lee DH, Sharma M (2019) The interpeduncular angle: a practical and objective marker for the detection and diagnosis of intracranial hypotension on brain MRI. Am J Neuroradiol 40:1299–1303. https://doi.org/10.3174/ajnr.a6120
Lakens D (2017) Equivalence Tests. Soc Psychol Personal Sci 8:355–362. https://doi.org/10.1177/1948550617697177
Lauzon C, Caffo B (2009) Easy multiplicity control in equivalence testing using two one-sided tests. Am Stat 63:147–154. https://doi.org/10.1198/tast.2009.0029
Anderson-Cook CM, Borror CM (2016) The difference between “equivalent” and “not different”. Qual Eng 28:249–262. https://doi.org/10.1080/08982112.2015.1079918
Koo TK, Li MY (2016) A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
R Core Team (2014) R: a language and environment for statistical computing
Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelso C (2014) Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 82:1449–1454. https://doi.org/10.1212/WNL.0000000000000342
Mirzayan MJ, Luetjens G, Borremans JJ, Regel JP, Krauss JK (2010) Extended long-term (> 5 years) outcome of cerebrospinal fluid shunting in idiopathic normal pressure hydrocephalus. Neurosurgery 67:295–301. https://doi.org/10.1227/01.NEU.0000371972.74630.EC
Giordan E, Palandri G, Lanzino G, Murad MH, Elder BD (2019) Outcomes and complications of different surgical treatments for idiopathic normal pressure hydrocephalus: a systematic review and meta-analysis. J Neurosurg 131:1024–1036. https://doi.org/10.3171/2018.5.JNS1875
Kahlon B (2002) Comparison between the lumbar infusion and CSF tap tests to predict outcome after shunt surgery in suspected normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 73:721–726. https://doi.org/10.1136/jnnp.73.6.721
Magdalinou NK, Ling H, Smith JDS, Schott JM, Watkins LD, Lees AJ (2013) Normal pressure hydrocephalus or progressive supranuclear palsy? A clinicopathological case series. J Neurol 260:1009–1013. https://doi.org/10.1007/s00415-012-6745-6
Hashimoto M, Ishikawa M, Mori E, Kuwana N (2010) Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 7:18. https://doi.org/10.1186/1743-8454-7-18
Kockum K, Virhammar J, Riklund K, Söderström L, Larsson EM, Laurell K (2019) Standardized image evaluation in patients with idiopathic normal pressure hydrocephalus: consistency and reproducibility. Neuroradiology 61:1397–1406. https://doi.org/10.1007/s00234-019-02273-2
Stezin A, Lenka A, Jhunjhunwala K, Saini J, Pal PK (2017) Advanced structural neuroimaging in progressive supranuclear palsy: where do we stand? Parkinsonism Relat Disord 36:19–32. https://doi.org/10.1016/j.parkreldis.2016.12.023
Pyatigorskaya N, Yahia-Cherif L, Gaurav R, Ewenczyk C, Gallea C, Valabregue R, Gargouri F, Magnin B, Degos B, Roze E, Bardinet E, Poupon C, Arnulf I, Vidailhet M, Lehericy S (2020) Multimodal magnetic resonance imaging quantification of brain changes in progressive supranuclear palsy. Mov Disord 35:161–170. https://doi.org/10.1002/mds.27877
Zhang K, Liang Z, Wang C, Zhang X, Yu B, Liu X (2019) Diagnostic validity of magnetic resonance parkinsonism index in differentiating patients with progressive supranuclear palsy from patients with Parkinson’s disease. Parkinsonism Relat Disord 66:176–181. https://doi.org/10.1016/j.parkreldis.2019.08.007
Nicoletti G, Caligiuri ME, Cherubini A, Morelli M, Novellino F, Arabia G, Salsone M, Quattrone A (2017) A fully automated, atlas-based approach for superior cerebellar peduncle evaluation in progressive supranuclear palsy phenotypes. Am J Neuroradiol 38:523–530. https://doi.org/10.3174/ajnr.A5048
Constantinides VC, Paraskevas GP, Velonakis G, Toulas P, Stefanis L, Kapaki E (2020) Midbrain morphology in idiopathic normal pressure hydrocephalus: a progressive supranuclear palsy mimic. Acta Neurol Scand 141:328–334. https://doi.org/10.1111/ane.13205
Karimi M, Perlmutter JS (2011) MRI measures predict progressive supranuclear palsy: clinically useful? Neurology 77:1028–1029. https://doi.org/10.1212/WNL.0b013e31822e14c7
Morelli M, Arabia G, Novellino F, Salsone M, Giofre L, Condino F, Messina D, Quattrone A (2011) MRI measurements predict PSP in unclassifiable parkinsonisms: a cohort study. Neurology 77:1042–1047. https://doi.org/10.1212/WNL.0b013e31822e55d0
Quattrone A, Morelli M, Williams DR, Vescio B, Arabia G, Nigro S, Nicoletti G, Salsone M, Novellino F, Nisticò R, Pucci F, Chiriaco C, Pugliese P, Bosco D, Caracciolo M (2016) MR parkinsonism index predicts vertical supranuclear gaze palsy in patients with PSP–parkinsonism. Neurology 87:1266–1273. https://doi.org/10.1212/WNL.0000000000003125
Picillo M, Tepedino MF, Abate F, Erro R, Ponticorvo S, Tartaglione S, Volpe G, Frosini D, Cecchi P, Cosottini M, Ceravolo R, Esposito F, Pellecchia MT, Barone P, Manara R (2020) Midbrain MRI assessments in progressive supranuclear palsy subtypes. J Neurol Neurosurg Psychiatry 91:98–103. https://doi.org/10.1136/jnnp-2019-321354
Acknowledgements
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Each author has contributed to all of the following areas:
- Conception and design, or acquisition of data, or analysis and interpretation of data
- Drafting the article or revising it critically for important intellectual content
- Final approval of the version to be published
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Requirement for informed consent was waived by the local IRBs (Comitato Etico Università Federico II, Naples, Italy; Comitato Etico Lazio 2, Rome, Italy).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
ESM 2
(XLSX 10 kb)
ESM 3
(XLSX 10 kb)
Supplementary Material
Fig. 4 (PNG 369 kb)
Supplementary Material
Fig. 5 (PNG 370 kb)
Supplementary Material
Fig. 6 (PNG 357 kb)
Supplementary Material
Fig. 7 (PNG 400 kb)
Supplementary Material
Fig. 8 (PNG 370 kb)
Supplementary Material
Fig. 9 (PNG 353 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ugga, L., Cuocolo, R., Cocozza, S. et al. Magnetic resonance parkinsonism indices and interpeduncular angle in idiopathic normal pressure hydrocephalus and progressive supranuclear palsy. Neuroradiology 62, 1657–1665 (2020). https://doi.org/10.1007/s00234-020-02500-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02500-1