Abstract
Introduction
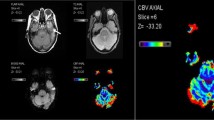
Our goal was to evaluate whether the T1 shortening effect caused by contrast leakage into brain tumors, a well-known confounding effect in the quantification of relative cerebral blood volume (rCBV) measurements, may be corrected by the administration of a predose of gadolinium-DTPA.
Methods
As part of their presurgical imaging protocol, 25 patients with primary brain tumors underwent two consecutive dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion MR studies. Intratumoral rCBV measurements and normalized rCBV values obtained during the first-pass and second-bolus studies were compared (Wilcoxon signed-ranks test). The frequency of relatively increased rCBV ratios on the second-bolus study was compared between enhancing and non-enhancing neoplasms (Fisher’s exact test). Postprocessing perfusion studies were evaluated for image quality on a scale of 0–3 (Wilcoxon signed-ranks test). Four studies were excluded due to unacceptable image quality.
Results
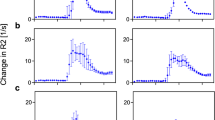
Mean normalized rCBVs were 9.04 (SD 4.64) for the first-pass and 7.99 (SD 3.84) for the second-bolus study. There was no statistically significant difference between the two perfusion studies in either intratumoral rCBV (P=0.237) or rCBV ratio (P=0.181). Five enhancing and four non-enhancing tumors showed a relative increase in rCBV ratio on the second-bolus study, without a significant difference between the groups. Image quality was not significantly different between perfusion studies.
Conclusion
Our results did not demonstrate a significant difference between first-pass and second-bolus rCBV measurements in DSC perfusion MR imaging. The administration of a predose of gadolinium-DTPA does not appear to be an efficient way of compensating for the underestimation of intratumoral rCBV values due to the T1 shortening effect.


Similar content being viewed by others
References
Chiang IC, Kuo YT, Lu CY, et al (2004) Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46:619–627
Provenzale JM, Wang GR, Brenner T, Petrella JR, Sorensen AG (2002) Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol 178:711–716
Aronen HJ, Gazit IE, Louis DN, et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Principi M, Italiani M, Guiducci A, et al (2003) Perfusion MRI in the evaluation of the relationship between tumour growth, necrosis and angiogenesis in glioblastomas and grade 1 meningiomas. Neuroradiology 45:205–211
Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F (2006) Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology 48:150–159
Essig M, Giesel F, Le-Huu M, Stieltjes B, von Tengg H, Weber MA (2004) Perfusion MRI in CNS disease: current concepts. Neuroradiology 46 [Suppl 2]:s201–s207
Knopp EA, Cha S, Johnson G, et al (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211:791–798
Sugahara T, Korogi Y, Kochi M, Ushio Y, Takahashi M (2001) Perfusion-sensitive MR imaging of gliomas: comparison between gradient-echo and spin-echo echo-planar imaging techniques. AJNR Am J Neuroradiol 22:1306–1315
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Nighoghossian N, Berthezene Y, Meyer R, et al (1997) Assessment of cerebrovascular reactivity by dynamic susceptibility contrast-enhanced MR imaging. J Neurol Sci 149:171–176
Levin JM, Kaufman MJ, Ross MH, et al (1995) Sequential dynamic susceptibility contrast MR experiments in human brain: residual contrast agent effect, steady state, and hemodynamic perturbation. Magn Reson Med 34:655–663
Levin JM, Wald LL, Kaufman MJ, Ross MH, Maas LC, Renshaw PF (1998) T1 effects in sequential dynamic susceptibility contrast experiments. J Magn Reson 130:292–295
Runge VM, Kirsch JE, Wells JW, Dunworth JN, Hilaire L, Woolfolk CE (1994) Repeat cerebral blood volume assessment with first-pass MR imaging. J Magn Reson Imaging 4:457–461
Wetzel SG, Cha S, Johnson G, et al (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224:797–803
Lev MH, Ozsunar Y, Henson JW, et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol 25:214–221
Schmainda KM, Rand SD, Joseph AM, et al (2004) Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol 25:1524–1532
Yang D, Korogi Y, Sugahara T, et al (2002) Cerebral gliomas: prospective comparison of multivoxel 2D chemical-shift imaging proton MR spectroscopy, echoplanar perfusion and diffusion-weighted MRI. Neuroradiology 44:656–666
Henry RG, Vigneron DB, Fischbein NJ, et al (2000) Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomas. AJNR Am J Neuroradiol 21:357–366
Law M, Oh S, Babb JS, et al (2006) Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging-prediction of patient clinical response. Radiology 238:658–667
Uematsu H, Maeda M, Sadato N, et al (2002) Measurement of the vascularity and vascular leakage of gliomas by double-echo dynamic magnetic resonance imaging: a preliminary study. Invest Radiol 37:571–576
Lev MH, Hochberg F (1998) Perfusion magnetic resonance imaging to assess brain tumor responses to new therapies. Cancer Control 5:115–123
Covarrubias DJ, Rosen BR, Lev MH (2004) Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist 9:528–537
Roberts HC, Roberts TP, Brasch RC, Dillon WP (2000) Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol 21:891–899
Hacklander T, Hofer M, Reichenbach JR, Rascher K, Furst G, Modder U (1996) Cerebral blood volume maps with dynamic contrast-enhanced T1-weighted FLASH imaging: normal values and preliminary clinical results. J Comput Assist Tomogr 20:532–539
Bruening R, Kwong KK, Vevea MJ, et al (1996) Echo-planar MR determination of relative cerebral blood volume in human brain tumors: T1 versus T2 weighting. AJNR Am J Neuroradiol 17:831–840
Uematsu H, Maeda M, Sadato N, et al (2001) Blood volume of gliomas determined by double-echo dynamic perfusion-weighted MR imaging: a preliminary study. AJNR Am J Neuroradiol 22:1915–1919
Uematsu H, Maeda M (2006) Double-echo perfusion-weighted MR imaging: basic concepts and application in brain tumors for the assessment of tumor blood volume and vascular permeability. Eur Radiol 16:180–186
Uematsu H, Maeda M, Sadato N, et al (2000) Vascular permeability: quantitative measurement with double-echo dynamic MR imaging – theory and clinical application. Radiology 214:912–917
Sugahara T, Korogi Y, Kochi M, et al (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 171:1479–1486
Sugahara T, Korogi Y, Shigematsu Y, et al (1999) Perfusion-sensitive MRI of cerebral lymphomas: a preliminary report. J Comput Assist Tomogr 23:232–237
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spampinato, M.V., Wooten, C., Dorlon, M. et al. Comparison of first-pass and second-bolus dynamic susceptibility perfusion MRI in brain tumors. Neuroradiology 48, 867–874 (2006). https://doi.org/10.1007/s00234-006-0134-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0134-8