Abstract
Introduction
The biological factors responsible for differential chemoresponsiveness in oligodendroglial tumours with or without the −1p/−19q genotype are unknown, but tumour vascularity may contribute. We aimed to determine whether dynamic susceptibility contrast (DSC) magnetic resonance imaging (MRI) could distinguish molecular subtypes of oligodendroglial tumour, and examined the relationship between relative cerebral blood volume (rCBV) and outcome following procarbazine, lomustine and vincristine (PCV) chemotherapy.
Methods
Pretherapy rCBV was calculated and inter- and intraobserver variability assessed. Allelic imbalance in 1p36, 19q13, 17p13, 10p12–15, and 10q22–26 and p53 mutation (exons 5–8) were determined. rCBV was compared with genotype and clinicopathological characteristics (n=37) and outcome following PCV chemotherapy (n=33).
Results
1p/19q loss was seen in 6/9 grade II oligodendrogliomas, 6/14 grade II oligoastrocytomas, 4/4 grade III oligodendrogliomas, and 3/10 grade III oligoastrocytomas. rCBV measurements had good inter- and intraobserver variability, but did not distinguish histology subtype or grade. Tumours with 1p/19q loss had higher rCBV values (Student’s t-test P=0.001). Receiver operating characteristic analysis revealed a cut-off of 1.59 for identifying genotype (sensitivity 92%, specificity 76%). Tumours with high and low rCBV showed response to chemotherapy. The −1p/−19q genotype, but not rCBV, was strongly associated with response, progression-free and overall survival following PCV chemotherapy. Tumours with high rCBV and intact 1p/19q were associated with shorter progression-free and overall patient survival than those with intact 1p/19q and low rCBV or high rCBV and 1p/19q loss.
Conclusion
rCBV identifies oligodendroglial tumours with 1p/19q loss, but does not predict chemosensitivity. The prognostic significance of rCBV may differ in oligodendroglial tumours with or without the −1p/−19q genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oligodendroglial tumours with combined loss of chromosomes 1p and 19q have a more indolent clinical evolution, respond more favourably to chemotherapy and show prolonged survival compared to those with intact 1p/19q, but the basis of these clinical differences is not well understood [1–5]. In addition, up to 30% of oligodendroglial neoplasms with intact 1p/19q may respond to chemotherapy [3, 6, 7], but the genetic and biological factors associated with response are unknown and not all patients with oligodendroglial tumours derive durable benefit from therapy. Assessment of the clinical utility of biological markers and non-invasive imaging techniques is essential to advance the clinical management of these patients.
Recently dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) has been used to calculate perfusion parameters in gliomas [8, 9], guide biopsy [10], provide prognostic information [11] and demonstrate differences in the vascularity of low-grade astrocytomas compared with oligodendrogliomas [8]. It has been suggested that increased vascularity and concomitant improved drug delivery may contribute to the chemoresponsiveness of oligodendroglial tumours, especially as lipophilic drugs (procarbazine, lomustine and temozolomide), which easily cross the blood–brain barrier, are used in the treatment of oligodendrogliomas [12–14]. Currently DSC-MRI is used in the radiological evaluation of gliomas, but its role in oligodendroglial neoplasms classified by genotype and the relationship with response and outcome following chemotherapy has not so far been investigated.
The aim of this study was to determine whether DSC-MRI obtained through routine pre-therapy MR diagnostic examinations may be used to distinguish molecular subtypes of oligodendroglial tumours, and to investigate the influence of tumour blood volume on outcome following procarbazine, lomustine and vincristine (PCV) chemotherapy compared to molecular genetics.
Methods
Patient selection
Patients for investigation were selected from those participating in a prospective study of adult oligodendroglial tumours eligible for chemotherapy at the Walton Centre for Neurology and Neurosurgery/Clatterbridge Centre for Oncology between May 2000 and June 2003 [7, 15–18]. The study had full ethical approval. For inclusion in this study, patients had to have (1) given research consent, (2) a histological diagnosis of oligodendroglioma or oligoastrocytoma based on the current World Health Organisation (WHO) classification [19], (3) a known 1p/19q status, and (4) undergone DSC-MRI before or after biopsy (2 image-guided; 35 serial stereotactic), but prior to commencing therapy. All patients (with primary and recurrent disease) underwent biopsy prior to PCV. Patients with post-resection imaging were excluded.
DCS-MRI
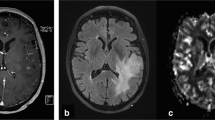
Imaging studies were obtained using a 1.5-T Signa MR scanner (General Electric Medical Systems). The MR imaging protocol consisted of T1-weighted spin-echo (SE) sagittal sections (400/14 TR/TE), axial fluid attenuated inversion recovery (FLAIR 8802/142/2200 TR/TE/TI), axial fast spin-echo (FSE) T2-weighted imaging (4360/102 TR/TE), 2D dynamic susceptibility-weighted, SE, echoplanar imaging (EPI 1900/80 ms, bandwidth 62.5) and postcontrast T1-weighted SE (600/14 TR/TE) or FLAIR (2280/11/750 TR/TE/TI) axial imaging. The T2-weighted FLAIR, FSE and postcontrast T1-weighted images were used to define regions of T2 hyperintensity and T1 enhancement. For the dynamic EPI series, the tumour size and location, and the position of the margins were determined from the T2-weighted FLAIR or FSE images to equally define low-grade and high-grade tumours. Nine slices were selected to cover the majority of the tumour volume. Slice thickness was 7 mm, with no gap. A standard dose of 0.1 mmol/kg body weight of dimeglumine gadopentetate (Gd DTPA) was injected intravenously manually at an approximate rate of 3 ml/s. A series of T2*-weighted multislice image sets (field of view 30×30, matrix 192×128) were acquired every 2 s during the first pass of the contrast agent. Image postprocessing was performed using commercial software (Functool2, version 6.0; Sun Microsystems). Negative enhancement integral (NEI) maps were generated for each patient relative to the signal intensity in the superior sagittal sinus (Fig. 1). The axial slice with the greatest cerebral blood volume and tumour area was chosen for analysis.
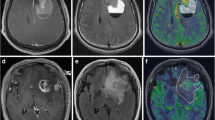
Calculation of rCBV using DSC-MR. CBV is derived from the drop in the T2* signal after injection of a bolus of paramagnetic compound [8, 9]. Using Functool2, the radiological tumour margin was delineated on the T2* sequence axial image and a single circular ROI placed over normal brain mirrored in the contralateral hemisphere (a, b). Negative enhancement integral (NEI) colour maps were generated (c, d) relative to the signal intensity in the superior sagittal sinus using image data between dynamic scans immediately before and after the contrast transient. CBV is proportional to the area under the contrast agent concentration-time curve and was calculated for each ROI by Functool2 using algorithms to integrate ΔR2i values (where ΔR2 is the change in the reciprocal of T2*). rCBV for each tumour was calculated from signal intensity time curves for individual ROIs placed within the delineated tumour region or in the large ROI in the contralateral hemisphere (as illustrated in e and f, arrows scans adjacent to the contrast transient) as described in the Materials and methods. a, c, e Low rCBV (0.73±0.17, mean±SD) in a grade II oligoastrocytoma with intact chromosomes 1p and 19q and; b, d, f high rCBV (4.15±0.87) in a grade II oligoastrocytoma with the −1p/−19q genotype
The radiological tumour margin was delineated on the T2* sequence axial image using a nongeometric region of interest (ROI), and a single circular region was placed over normal brain mirrored in the contralateral hemisphere. Depending on tumour location, the mirrored area could include both white and grey matter. ROIs were placed according to published methods giving the lowest inter- and intraobserver variability [20] within the delineated tumour region. Briefly, five ROIs (area range 12–27 mm2) were placed over the tumour areas with the highest blood volume. Cystic and hemorrhagic regions were avoided and care was taken not to place ROIs over normal blood vessels or a biopsy tract where seen. Three independent analyses were performed by a neuroradiologist (T.S.S.) and a neurosurgeon (M.D.J.) blinded to histology and genotype, to assess inter- and intraobserver variability. For each observation, data were expressed relative to the internal reference (four ROIs placed within the delineated mirrored region of normal brain without deliberate bias towards either grey or white matter) to generate relative cerebral blood volume (rCBV) values (mean ROItumour/mean ROInormal brain) and accommodate physiological differences between patients [21]. For comparison with clinical data, the mean rCBV from these three data sets was used.
Molecular genetics
For each patient regions of tumour histology in pretherapy biopsy specimens (formalin-fixed, paraffin-embedded tissues or intraoperative diagnostic smear preparations) representative of the most aggressive tissue available and the overall pathology diagnosis were selected for laser capture microdissection to enrich the tumour component in the samples for analysis. Allelic imbalance was investigated using paired normal (microdissected uninvolved brain or DNA from blood) and tumour tissue samples and multiple simultaneous PCR amplification of a panel of microsatellite markers, capillary electrophoresis and data analysis as described previously [16–18]. The microsatellite panel included: chromosome 1 – D1S2667, D1S508, D1S214 (1p36); chromosome 19 – D19S412, D19S112, D19S596 (19q13); chromosome 17 – D17S796, D17S1176, D17S1353 (17p13); chromosome 10p – D10S89, D10S179 (10p12-10p15); chromosome 10q – D10S1687, D10s2491, D10S583, D10S587, D10S212 (10q22-10q26). Additional markers, D1S468, D1S2795 (1p36) and D19S217, D19S572 (19q13) were used in cases that lacked informativity for given loci. p53 mutations (exons 5–8) were detected by direct sequence analysis of PCR products.
PCV chemotherapy, response and clinical outcome
All patients receiving oncology therapy had evidence of advancing disease (either radiographic or clinical progression) prior to therapy. Patients with mass effect or evidence of malignancy were treated at presentation; others were followed clinically until progression. Oncology therapy was based on individual patient’s clinical status/choice. Patients fit for PCV were offered PCV chemotherapy, or radiotherapy if fertility was an issue. PCV chemotherapy was administered according to standard clinical protocols [22]: lomustine (CCNU) (110 mg/m2), procarbazine (60 mg/m2) and vincristine (1.4 mg/m2, maximum 2 mg) were given on days 1, 8–21, and 8 and 29. respectively. Cycles were repeated every 6 weeks for a maximum of six cycles. As described previously [18], a consultant neuroradiologist reviewed all available MR or CT images taken before, during and after chemotherapy, and at follow-up. The largest perpendicular diameters of the tumour were measured on axial sections of T2-weighted MR images or contrast-enhanced regions on T1-weighted MR or CT images. Response was assessed using the criteria of Macdonald et al. [23] and postcontrast T1-weighted MRI in 25enhancing tumours. In tumours showing no enhancement (n=6) or in those in which contrast enhancement was not assessable (n=1), response was based on T2-weighted images.
Information regarding current management, follow-up and outcome was collected prospectively. Progression-free survival (PFS) and overall survival (OS) were calculated from the start of PCV to progression, last seen, or death as appropriate.
Statistical analysis
Inter- and intraobserver variability was determined using Bland and Altman plots [24]. Differences in rCBV between genetic and clinical factors were assessed using Student’s t-test for numerical data and the Chi-squared or Fisher’s exact test for categorical data. Receiver operator characteristics (ROC) analysis was performed to define the optimal rCBV cut-off for predicting 1p/19q status. Kaplan-Meier survival curves were obtained and differences in OS or PFS were tested for statistical significance using the Log Rank test. Cox regression multivariate analysis of factors shown to be significant in univariate analysis was by forward stepwise entry of parameters at a significance of 0.05 for entry and 0.01 for removal. Probability (P) values less than 0.05 were considered significant.
Results
The study included 30 patients with primary tumours without previous therapy and 7 with recurrent or persistent disease following radiotherapy. Median time between previous radiotherapy and MR imaging was 6.5 years (range 1.1–14.2 years). Median age was 44 years (range 28–71 years).
Interobserver and intraobserver variability
Tumour rCBV was obtained from DSC-MRI (Fig. 1) by two independent observers. Bland and Altman plots revealed low inter- and intraobserver variation (Fig. 2). The closest observed limits of agreement were between the first observations of the neuroradiologist and neurosurgeon (mean −0.17, range −1.27 to 0.93; Fig. 2a). The widest observed limits of agreement were between the repeat observations of the neurosurgeon (mean 0.08, range −1.59 to 1.95; Fig. 2c). The greatest variation was observed in tumours with higher rCBV values.
Histopathology, genotype and rCBV
Oligodendroglial tumours of different histopathology subtypes and grade did not have significantly different rCBV measurements (Figure 3a,b). Loss of both 1p36 and 19q13 was seen in 6/9 grade II oligodendrogliomas, 6/14 grade II oligoastrocytomas, 4/4 grade III oligodendrogliomas, and 3/10 grade III oligoastrocytomas. Tumours with the −1p/−19q genotype had significantly higher rCBV than those with intact 1p and 19q (Fig. 3c). Similar findings were observed when primary tumours only were analysed (data not shown). ROC analysis was used to determine the specificity and sensitivity of rCBV as a potential non-invasive tool to distinguish oligodendroglial neoplasms with or without the −1p/−19q genotype. The area under the ROC curve was 0.853 (95% confidence interval: 0.778–0.927). A cut-off value of 1.59 for rCBV (sensitivity 92%, specificity 76%) best discriminated oligodendroglial tumours with and without the −1p/−19q genotype (Fig. 3c).
To facilitate comparison of rCBV with genotype and clinicopathological characteristics (Table 1), tumours were considered to have high rCBV if this was greater than 1.59 as determined by the ROC analysis (Fig. 4). High rCBV values were significantly more common in tumours with loss of 1p36 and 19q13 and less common in tumours with 17p13 loss and p53 mutation. Three tumours in the series had loss of chromosome 10q22–26, of which two also had loss of 10p11–15; all had high rCBV. One tumour with high rCBV had loss of 1p36 without loss of 19q13. As for numerical data, there was no significant association between high rCBV and histology subtype or grade. Contrast enhancement was weakly associated with high rCBV in the series but not in primary tumours. No associations were seen between tumour rCBV and patient age <50 years versus age ≥50 years, gender or temporal versus non-temporal location. Similar associations between rCBV, genotype and clinicopathological characteristics were obtained if only primary tumours were analysed (Table 1).
rCBV, molecular genetics and clinical characteristics. rCBV for each tumour is given as the mean±standard deviation of three independent observations. The arrow indicates tumours with rCBV above (high rCBV) and below the cut-off value of 1.59 (low rCBV). CR complete response (disappearance of all tumour, off steroids and neurologically stable or improved), PR partial response (50% or greater reduction in cross-sectional area, steroids stable or reduced, and neurologically stable or improved); MR minor response (>25 to <50% reduction in cross-sectional area, steroids stable or reduced, and neurologically stable or improved); PD progressive disease (25% or greater increase in cross-sectional area or any new tumour on CT/MR images and/or neurologically worse with steroids stable or increased); SD stable disease (all other situations); P primary tumour, R recurrent tumour
rCBV and outcome following PCV chemotherapy
Of the 37 patients, 33 had PCV chemotherapy following DSC-MRI (median 0.7 months, range 0.1–15.2 months), of whom 26 with primary tumours received PCV chemotherapy as the first oncology therapy, 6 with recurrent or persistent disease treated initially with radiotherapy received PCV as second oncology therapy, and 1 had PCV following radiotherapy and temozolomide. One patient with primary tumour had therapy switched to radiotherapy at the end of the first cycle because of reaction to procarbazine. The remainder received a median of four cycles of PCV (range one to six). Response to therapy was assessed in 32 patients (Fig. 4). Measured radiological changes and clinical factors permitted response assessment using standard criteria of Macdonald et al. [23] in all patients showing enhancement. However, in tumours showing no enhancement assessed using T2-weighted MR images, three showed radiological change in cross-sectional area of >25% to <50%, accompanied by clinical benefit; these were included in the minor response (MR) category. None of those showing enhancement had measured radiological change in this range. Response (i.e. complete, partial or minor; see Fig. 4 legend for definition) was seen in tumours with and without high rCBV. Similarly, non-responders (i.e. patients with stable or progressive disease) had either high or low tumour rCBV (Table 1, Fig. 4). Response was strongly associated with genotype; all 16 tumours with loss of 1p36 and 19q13 responded compared with 5 of 17 tumours with intact 1p36/19q13 (Chi2 P<0.001). Of the 16 responders in the 1p36/19q13 loss group, 14 had high rCBV. The five responders in the intact 1p36/19q13 group all had low rCBV and the four tumours with high rCBV failed to respond.
The relationship between rCBV, genotype, histopathology and survival following PCV in the series and in those with primary tumour is given in Table 2. The −1p/−19q genotype and low-grade pathology were associated with longer PFS and OS. Histopathology subtype was not significantly associated with outcome. Increased rCBV was only associated with short PFS and OS when adjusted for the −1p/−19q genotype. Tumours with intact 1p/19q and high rCBV (two grade III primary oligoastrocytoma, one grade II recurrent oligodendroglioma, one grade III recurrent oligoastrocytoma) were associated with shorter PFS and OS than tumours with intact 1p/19q and low rCBV or those with lp/19q loss and high rCBV (Fig. 5a,b). Similar findings were observed if primary tumour only were analysed (Fig. 5c,d). Compared with significant clinicopathological parameters, high rCBV was an independent prognostic factor for PFS when adjusted for 1p/19q status (Cox regression: PFS: hazard ratio 13.4, 95% CI 1.3–133; P=0.008) and for PFS and OS in patients with intact 1p/19q (Cox regression: PFS: hazard ratio 16.5, 95% CI 1.8–152.2; P=0.004; OS: hazard ratio 7.4, 95% CI 1.6–33.3; P=0.013).
rCBV and outcome following PCV chemotherapy: a, b all patients; c, d patients with primary tumour only; a, c progression-free survival; b, d overall survival.① Patients with tumours with low rCBV (<1.59) and loss of 1p36 and 19q13 (all n=2, primary n=2).② Patients with tumours with high rCBV (>1.59) and loss of 1p36 and 19q13 (all n=15, primary n=12).③ Patients with tumours with low rCBV (<1.59) with intact 1p36 and 19q13 (all n=12, primary n=10).④ Patients with tumours with high rCBV (>1.59) and intact 1p36 and 19q13 (all n=4, primary n=2)
Discussion
To our knowledge this is the first study investigating the relationship between rCBV, genotype and outcome in oligodendroglial tumours treated with PCV chemotherapy. The cohort was drawn from a larger series in an ongoing prospective study of oligodendroglial neoplasms [15–18] and reflects the range of patients given PCV chemotherapy in routine clinical practice. Tumours with rCBV >1.59 were more likely to have 1p/19q loss. PCV chemosensitivity was predicted by 1p/19q loss, but high rCBV was not necessary for response. Following chemotherapy, rCBV was significantly associated with PFS and OS only after adjustment for genotype.
DSC-MRI can be used to determine cerebral perfusion parameters such as rCBV, relative cerebral blood flow and permeability, but with the instrumentation available to us was restricted to rCBV. As in other studies [8, 20, 25, 26], placement of ROI in regions within tumour of greatest signal intensity yielded data with good inter- and intraobserver variability. The greatest variation was seen in tumours with high rCBV, reflecting heterogeneity of blood volume within these tumours. In this series, tumours with the −1p/−19q genotype were more likely to have high rCBV than those with intact 1p/19q. The ROC analysis determined an optimal cut-off (rCBV >1.59) for predicting the −1p/−19q genotype with 92% sensitivity and 76% specificity. These data suggest that in conjunction with histopathology, rCBV may be used to distinguish tumours of the oligodendroglial lineage.
MRI studies of tumour vasculature in gliomas have demonstrated that rCBV may be useful in the non-invasive determination of tumour grade and vascularity [21, 25, 27, 28]. Compared to their low-grade counterparts, high-grade tumours have elevated rCBV, associated with increased mitotic activity [29]. rCBV correlates with conventional angiographic assessment of tumour vascular density and histological measures of angiogenesis such as microvascular density and VEGF expression [26, 30–32], which reflects the histopathological finding of increasing neovascularization with grade [19]. However, most studies have investigated astrocytic gliomas or included a variety of glioma pathologies and large series of oligodendroglial neoplasms are uncommon. Recent reports suggest that rCBV values in oligodendrogliomas render DSC-MRI-based tumour grading inaccurate [28, 33], and rCBV may be similar in low- and high-grade oligodendrogliomas [33]. Indeed low-grade oligodendrogliomas have significantly elevated rCBV compared to low-grade astrocytomas [8]. In our series, rCBV did not distinguish oligodendrogliomas from oligoastrocytomas, nor was rCBV significantly different between grade II and III tumours. This lack of correlation between rCBV and grade is consistent with the increased microvessel density seen in both low- and high-grade oligodendroglial tumours [34, 35].
It has been postulated that the tumour vasculature in oligodendrogliomas contributes to their chemosensitivity [12–14]. In an anaplastic oligodendroglioma treated with PCV chemotherapy, post-therapy volume changes and normalization of rCBV were more evident in regions of the pretherapy tumour that showed high rCBV [13]. In the present study, tumours with and without the −1p/−19q genotype, and high or low rCBV showed response to chemotherapy when assessed using conventional criteria [1, 23]. These data indicate that rCBV measured through DSC-MRI is not a key factor in determining PCV chemosensitivity. These findings may reflect the limitations of DSC-MRI associated with contrast recirculation and disruption of the blood–brain barrier in tumours [8, 9]. Alternatively, chemosensitivity may be influenced more by blood flow than by volume. Further studies to measure additional parameters associated with vascular perfusion, including blood flow and permeability are essential to establish whether these factors contribute to chemosensitivity.
The −1p/−19q genotype is known to be associated with longer PFS and OS [5] but the impact of tumour vasculature on prognosis in oligodendrogliomas is confined to a few histopathological [34, 36, 37] and imaging studies [12]. Although our cohort is small, we have presented preliminary data that the prognostic significance of rCBV may differ in tumours with or without the −1p/−19q genotype. 1p/19q loss and low-grade pathology were associated with favourable PFS and OS following PCV. In other studies of astrocytic gliomas [28] or mixed low-grade gliomas [11], high rCBV predicted poor prognosis. In our series, rCBV alone was an unreliable indicator of outcome, showing prognostic significance only after stratification for genotype, suggesting different effects for the two genetic subgroups at the cut-off used to classify tumours with high rCBV. With this cut-off, tumours with high rCBV and 1p/19q loss were associated with longer survival than those with high rCBV and intact 1p/19q. Unlike astrocytic gliomas, high rCBV values do not necessarily indicate aggressive biology associated with poor outcome in oligodendroglial neoplasms with 1p/19q loss, suggestive of differences in the baseline biology of these two genetic subtypes. These preliminary data therefore indicate that further investigation of the prognostic significance of rCBV in molecular subtypes of glioma in a much larger series is warranted.
Conclusion
In conclusion, we have shown that high rCBV is more likely in oligodendroglial tumours with the −1p/−19q genotype and may be a useful aid to diagnosis; however, rCBV does not predict chemosensitivity. Lack of correlation with histopathology grade and the data suggesting that the prognostic significance of rCBV may differ in patients with or without the −1p/−19q genotype, indicates that different interpretation of rCBV data may be necessary for oligodendroglial compared with astrocytic gliomas. Further study is essential to fully evaluate the role of DSC-MRI in the non-invasive diagnosis and prediction of prognosis in gliomas with an oligodendroglial phenotype.
References
Buckner JC, Gesme D Jr, O’Fallon JR et al (2003) Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol 21:251–255
Cairncross JG, Ueki K, Zlatescu MC et al (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
Ino Y, Betensky RA, Zlatescu MC et al (2001) Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 7:839–845
Smith JS, Perry A, Borell TJ, et al (2000) Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 18:636–645
van den Bent MJ (2004) Advances in the biology and treatment of oligodendrogliomas. Curr Opin Neurol 17:675–680
Sasaki H, Zlatescu MC, Betensky RA et al (2002) Histopathological-molecular genetic correlations in referral pathologist-diagnosed low-grade “oligodendroglioma”. J Neuropathol Exp Neurol 61:58–63
Walker C, Smith TS, Haylock B et al (2006) Clinical use of genotype to predict chemosensitivity in oligodendroglial tumors. Neurology 66:1661–1667
Cha S, Tihan T, Crawford F et al (2005) Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 26:266–273
Keston P, Murray AD, Jackson A (2003) Cerebral perfusion imaging using contrast-enhanced MRI. Clin Radiol 58:505–513
Cha S, Knopp EA, Johnson G et al (2002) Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology 223:11–29
Law M, Oh S, Babb JS et al (2006) Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging–prediction of patient clinical response. Radiology 238:658–667
Warnke PC, Berlis A, Ostertag C (1998) Physiological characterization of malignant oligodendrogliomas responding to PCV chemotherapy. J Neurosurg 88:399A
Jager HR, Waldman AD, Benton C, Fox N, Rees J (2005) Differential chemosensitivity of tumor components in a malignant oligodendroglioma: assessment with diffusion-weighted, perfusion-weighted, and serial volumetric MR imaging. AJNR Am J Neuroradiol 26:274–278
Godfraind C, Rousseau E, Ruchoux MM, Scaravilli F, Vikkula M (2003) Tumour necrosis and microvascular proliferation are associated with 9p deletion and CDKN2A alterations in 1p/19q-deleted oligodendrogliomas. Neuropathol Appl Neurobiol 29:462–471
Jenkinson MD, Smith TS, Joyce K et al (2005) MRS of oligodendroglial tumors: correlation with histopathology and genetic subtypes. Neurology 64:2085–2089
Walker C, du Plessis DG, Fildes D et al (2004) Correlation of molecular genetics with molecular and morphological imaging in gliomas with an oligodendroglial component. Clin Cancer Res 10:7182–7191
Walker C, du Plessis DG, Joyce KA et al (2005) Molecular pathology and clinical characteristics of oligodendroglial neoplasms. Ann Neurol 57:855–865
Walker C, Haylock B, Husband D et al (2006) Clinical use of genotype to predict chemosensitivity in oligodendroglial tumors. Neurology 66:1661–1667
Kleihues P, Cavanee W (eds) (2000) Pathology and genetics of tumours of the nervous system. IARC Press, Lyon
Wetzel SG, Cha S, Johnson G et al (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224:797–803
Warmuth C, Gunther M, Zimmer C (2003) Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology 228:523–532
Levin VA, Edwards MS, Wright DC et al (1980) Modified procarbazine, CCNU, and vincristine (PCV 3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat Rep 64:237–244
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Maia AC Jr, Malheiros SM da Rocha AJ, et al (2005) MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol 26:777–783
Hakyemez B, Erdogan C, Ercan I et al (2005) High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol 60:493–502
Lev MH, Ozsunar Y, Henson JW et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol 25:214–221
Roberts HC, Roberts TP, Bollen AW et al (2001) Correlation of microvascular permeability derived from dynamic contrast-enhanced MR imaging with histologic grade and tumor labeling index: a study in human brain tumors. Acad Radiol 8:384–391
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Aronen HJ, Pardo FS, Kennedy DN et al (2000) High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin Cancer Res 6:2189–2200
Sugahara T, Korogi Y, Kochi M et al (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 171:1479–1486
Xu M, See SJ, Ng WH et al (2005) Comparison of magnetic resonance spectroscopy and perfusion-weighted imaging in presurgical grading of oligodendroglial tumors. Neurosurgery 56:919–926; discussion 919–926
Birner P, Gatterbauer B, Oberhuber G et al (2001) Expression of hypoxia-inducible factor-1 alpha in oligodendrogliomas: its impact on prognosis and on neoangiogenesis. Cancer 92:165–171
Christov C, Adle-Biassette H, Le Guerinel C, Natchev S, Gherardi RK (1998) Immunohistochemical detection of vascular endothelial growth factor (VEGF) in the vasculature of oligodendrogliomas. Neuropathol Appl Neurobiol 24:29–35
Schiffer D, Bosone I, Dutto A, Di Vito N, Chio A (1999) The prognostic role of vessel productive changes and vessel density in oligodendroglioma. J Neurooncol 44:99–107
Vaquero J, Zurita M, Morales C, Coca S (2002) Prognostic significance of tumor-enhancement and angiogenesis in oligodendroglioma. Acta Neurol Scand 106:19–23
Acknowledgements
The study was supported by fellowship grants from The Royal Colleges of Surgeons of Edinburgh and Ireland, and Clatterbridge Cancer Research Trust. This research was made possible through the support of the Walton Centre for Neurology and Neurosurgery and Clatterbridge Centre for Oncology. We thank Dr. D.R. Sibson, Clatterbridge Cancer Research Trust, for helpful support and discussion.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Jenkinson, M.D., Smith, T.S., Joyce, K.A. et al. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology 48, 703–713 (2006). https://doi.org/10.1007/s00234-006-0122-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0122-z