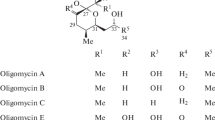
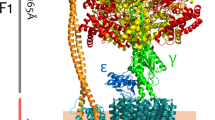
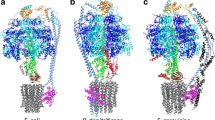
Abstract
The F1FO-ATP synthase is the only enzyme in nature endowed with bi-functional catalytic mechanism of synthesis and hydrolysis of ATP. The enzyme functions, not only confined to energy transduction, are tied to three intrinsic features of the annular arrangement of c subunits which constitutes the so-called c-ring, the core of the membrane-embedded FO domain: (i) the c-ring constitution is linked to the number of ions (H+ or Na+) channeled across the membrane during the dissipation of the transmembrane electrochemical gradient, which in turn determines the species-specific bioenergetic cost of ATP, the “molecular currency unit” of energy transfer in all living beings; (ii) the c-ring is increasingly involved in the mitochondrial permeability transition, an event linked to cell death and to most mitochondrial dysfunctions; (iii) the c subunit species-specific amino acid sequence and susceptibility to post-translational modifications can address antibacterial drug design according to the model of enzyme inhibitors which target the c subunits. Therefore, the simple c-ring structure not only allows the F1FO-ATP synthase to perform the two opposite tasks of molecular machine of cell life and death, but it also amplifies the enzyme’s potential role as a drug target.



Similar content being viewed by others
References
Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr, Jonas EA (2014) An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA 111:10580–10585. doi:10.1073/pnas.1401591111
Allegretti M, Klusch N, Mills DJ, Vonck J, Kühlbrandt W, Davies KM (2015) Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase. Nature 521:237–240. doi:10.1038/nature14185
Andries K, Verhasselt P, Guillemont J, Göhlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V (2005) A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227
Antoniel M, Giorgio V, Fogolari F, Glick GD, Bernardi P, Lippe G (2014) The oligomycin-sensitivity conferring protein of mitochondrial ATP synthase: emerging new roles in mitochondrial pathophysiology. Int J Mol Sci 15:7513–7536. doi:10.3390/ijms15057513
Azarashvili T, Odinokova I, Bakunts A, Ternovsky V, Krestinina O, Tyynelä J, Saris NE (2014) Potential role of subunit c of F0F1-ATPase and subunit c of storage body in the mitochondrial permeability transition. Effect of the phosphorylation status of subunit c on pore opening. Cell Calcium 55:69–77. doi:10.1016/j.ceca.2013.12.002
Azzone GF, Azzi A (1965a) Volume changes in liver mitochondria. Proc Natl Acad Sci USA 53:1084–1089
Azzone GF, Azzi A (1965b) Volume changes induced by inorganic phosphate in liver mitochondria. Biochem J 94:10C–11C
Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9:550–555
Balemans W, Vranckx L, Nounis N, Pop O, Guillermont J, Vergauwen K, Mol S, Glissen R, Motte M, Lancois D, De Bolle M, Bonroy K, Lill H, Andries K, Bald D, Koul A (2012) Novel antibiotics targeting respiratory ATP synthesis in Gram-positive pathogenic bacteria. Antimicrob Agents Chemother 56:4131–4139
Bernardi P, Di Lisa F (2015) The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol 78:100–106. doi:10.1016/j.yjmcc.2014.09.023
Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA (2006) The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 273:2077–2099
Bernardi P, Di Lisa F, Fogolari F, Lippe G (2015) From ATP to PTP and back: a dual function for the mitochondrial ATP synthase. Circ Res 116:1850–1862
Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P (2013) Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12:674–683. doi:10.4161/cc.23599
Bonora M, Wieckowski MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 34:1608. doi:10.1038/onc.2014.462
Boyer PD (1997) The ATP synthase—a splendid molecular machine. Annu Rev Biochem 66:717–749
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312. doi:10.1042/BJ20110162
Chen C, Ko Y, Delannoy M, Ludtke SJ, Chiu W, Pedersen PL (2004) Mitochondrial ATP synthasome: three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J Biol Chem 279:31761–31768
Crompton M, Ellinger H, Costi A (1988) Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255:357–360
Dimroth P, von Ballmoos C, Meier T (2006) Catalytic and mechanical cycles in F-ATP synthases. EMBO Rep 7:276–282
Elston T, Wang H, Oster G (1998) Energy transduction in ATP synthase. Nature 391:510–513
Faccenda D, Tan CH, Seraphim A, Duchen MR, Campanella M (2013) IF1 limits the apoptotic-signalling cascade by preventing mitochondrial remodelling. Cell Death Differ 20:686–697. doi:10.1038/cdd.2012.163
Ferguson SJ (2010) ATP synthase: from sequence to ring size to the P/O ratio. Proc Natl Acad Sci USA 107:16755–16756. doi:10.1073/pnas.1012260107
Galanis M, Mattoon JR, Nagley P (1989) Amino acid substitutions in mitochondrial ATP synthase subunit 9 of Saccharomyces cerevisiae leading to venturicidin or ossamycin resistance. FEBS Lett 249:333–336
Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G (2009) Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem 284:33982–33988. doi:10.1074/jbc.M109.020115
Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G, Bernardi P (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA 110:5887–5892. doi:10.1073/pnas.1217823110
Giraud MF, Paumard P, Sanchez C, Brèthes D, Velours J, Dautant A (2012) Rotor architecture in the yeast and bovine F1-c-ring complexes of F-ATP synthase. J Struct Biol 177:490–497. doi:10.1016/j.jsb.2011.10.015
Haagsma AC, Abdillahi-Ibrahim R, Wagner MJ, Krab K, Vergauwen K, Guillemont J, Andries K, Lill H, Koul A, Bald D (2009) Selectivity of TMC207 towards mycobacterial ATP synthase compared with that towards the eukaryotic homologue. Antimicrob Agents Chemother 53:1290–1292. doi:10.1128/AAC.01393-08
Halestrap AP (2014) The C ring of the F1Fo ATP synthase forms the mitochondrial permeability transition pore: a critical appraisal. Front Oncol. 4:234. doi:10.3389/fonc.2014.00234
Hong S, Pedersen PL (2008) ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol Mol Biol Rev 72:590–641. doi:10.1128/MMBR.00016-08
Hunter DR, Haworth RA (1979) The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys 195:453–459
Junge W, Müller DJ (2011) Biochemistry. Seeing a molecular motor at work. Science 333:704–705. doi:10.1126/science.1210238
Junge W, Sielaff H, Engelbrecht S (2009) Torque generation and elastic power transmission in the rotary F(O)F(1)-ATPase. Nature 459:364–370. doi:10.1038/nature08145
Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J, Kinnally KW, Molkentin JD (2013) Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2:e00772. doi:10.7554/eLife.00772
Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427:461–465
Koul A, Dendouga N, Vergauwen K, Molenberghs B, Vranckx L, Willebrords R, Ristic Z, Lill H, Dorange I, Guillemont J, Bald D, Andries K (2007) Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol 3:323–324
Lakshamanan M, Xavier AS (2013) Bedaquiline-the first ATPsynthase inhibitor against multi-drug resistant tuberculosis. J Young Pharm 5:112–115. doi:10.1016/j.jyp.2013.12.002
Lehninger AL (1959) Reversal of various types of mitochondrial swelling by adenosine triphosphate. J Biol Chem 234:2465–2471
Matthies D, Zhou W, Klyszejko AL, Anselmi C, Yildiz Ö, Brandt K, Müller V, Faraldo-Gómez JD, Meier T (2014) High-resolution structure and mechanism of an F/V-hybrid rotor ring in a Na+-coupled ATP synthase. Nat Commun. doi:10.1038/ncomms6286
Meier T, Polzer P, Diederichs K, Welte W, Dimroth P (2005) Structure of the rotor ring of F-type Na+-ATPase from Ilyobacter tartaricus. Science 308:659–662
Mitome N, Ono S, Sato H, Suzuki T, Sone N, Yoshida M (2010) Essential arginine residue of the F(o)-a subunit in F(o)F(1)-ATP synthase has a role to prevent the proton shortcut without c-ring rotation in the F(o) proton channel. Biochem J 430:171–177. doi:10.1042/BJ20100621
Nagley P, Hall RM, Ooi BG (1986) Amino acid substitutions in mitochondrial ATPase subunit 9 of Saccharomyces cerevisiae leading to oligomycin or venturicidin resistance. FEBS Lett 195:159–163
Nathanson L, Gromet-Elhanan Z (2000) Mutations in the beta-subunit Thr(159) and Glu(184) of the Rhodospirillum rubrum F(0)F(1) ATP synthase reveal differences in ligands for the coupled Mg(2+)- and decoupled Ca(2+)-dependent F(0)F(1) activities. J Biol Chem 275:901–905
Nesci S, Ventrella V, Trombetti F, Pirini M, Pagliarani A (2011) Multi-site TBT binding skews the inhibition of oligomycin on the mitochondrial Mg-ATPase in Mytilus galloprovincialis. Biochimie 93:1157–1164. doi:10.1016/j.biochi.2011.04.008
Nesci S, Ventrella V, Trombetti F, Pirini M, Pagliarani A (2012) Tri-n-butyltin binding to a low-affinity site decreases the F1FO-ATPase sensitivity to oligomycin in mussel mitochondria. Appl Organomet Chem 26:593–599. doi:10.1002/aoc.2904
Nesci S, Ventrella V, Pagliarani A (2013a) Modulation of the F1FO-ATPase function by butyltin compounds. Appl Organomet Chem 27:199–205. doi:10.1002/aoc.2948
Nesci S, Ventrella V, Trombetti F, Pirini M, Pagliarani A (2013b) Mussel and mammalian ATP synthase share the same bioenergetic cost of ATP. J Bioenerg Biomembr 45:289–300. doi:10.1007/s10863-013-9504-1
Nesci S, Ventrella V, Trombetti F, Pirini M, Pagliarani A (2014a) Thiol oxidation of mitochondrial FO-c subunits: a way to switch off antimicrobial drug targets of the mitochondrial ATP synthase. Med Hypotheses 83:160–165. doi:10.1016/j.mehy.2014.05.004
Nesci S, Ventrella V, Trombetti F, Pirini M, Pagliarani A (2014b) The mitochondrial F1FO-ATPase desensitization to oligomycin by tributyltin is due to thiol oxidation. Biochimie 97:128–137. doi:10.1016/j.biochi.2013.10.002
Nesci S, Ventrella V, Trombetti F, Pirini M, Pagliarani A (2014c) Thiol oxidation is crucial in the desensitization of the mitochondrial F1FO-ATPase to oligomycin and other macrolide antibiotics. Biochim Biophys Acta 1840:1882–1891. doi:10.1016/j.bbagen.2014.01.008
Nesci S, Trombetti F, Ventrella V, Pagliarani A (2015) Opposite rotation directions in the synthesis and hydrolysis of ATP by the ATP synthase: hints from a subunit asymmetry. J Membr Biol 248:163–169. doi:10.1007/s00232-014-9760-y
Nicholls DG, Ferguson SJ (2013) Bioenergetics, 4th edn. Academic Press, Amsterdam
Oberfeld B, Brunner J, Dimroth P (2006) Phospholipids occupy the internal lumen of the c ring of the ATP synthase of Escherichia coli. Biochemistry 45:1841–1851
Pagliarani A, Nesci S, Ventrella V (2013) Modifiers of the oligomycin sensitivity of the mitochondrial F1FO-ATPase. Mitochondrion 13:312–319. doi:10.1016/j.mito.2013.04.005
Papageorgiou S, Melandri AB, Solaini G (1998) Relevance of divalent cations to ATP-driven proton pumping in beef heart mitochondrial F0F1-ATPase. J Bioenerg Biomembr 30:533–541
Petersen J, Förster K, Turina P, Gräber P (2012) Comparison of the H+/ATP ratios of the H+-ATP synthases from yeast and from chloroplast. Proc Natl Acad Sci USA 109:11150–11155. doi:10.1073/pnas.1202799109
Pogoryelov D, Yu J, Meier T, Vonck J, Dimroth P, Müller DJ (2005) The c15 ring of the Spirulina platensis F-ATP synthase: F1/Fo symmetry mismatch is not obligatory. EMBO Rep 6:1040–1044
Pogoryelov D, Yildiz O, Faraldo-Gómez JD, Meier T (2009) High-resolution structure of the rotor ring of a proton-dependent ATP synthase. Nat Struct Mol Biol 16:1068–1073. doi:10.1038/nsmb.1678
Pogoryelov D, Krah A, Langer JD, Yildiz Ö, Faraldo-Gómez JD, Meier T (2010) Microscopic rotary mechanism of ion translocation in the F(o) complex of ATP synthases. Nat Chem Biol 6:891–899
Preiss L, Yildiz O, Hicks DB, Krulwich TA, Meier T (2010) A new type of proton coordination in an F(1)F(o)-ATP synthase rotor ring. PLoS Biol 8:e1000443. doi:10.1371/journal.pbio.1000443
Preiss L, Klyszejko AL, Hicks DB, Liu J, Fackelmayer OJ, Yildiz Ö, Krulwich TA, Meier T (2013) The c-ring stoichiometry of ATP synthase is adapted to cell physiological requirements of alkaliphilic Bacillus pseudofirmus OF4. Proc Natl Acad Sci USA 110:7874–7879. doi:10.1073/pnas.1303333110
Preiss L, Langer JD, Yildiz Ö, Eckhardt-Strelau L, Guillemont JEG, Koul A, Meier T (2015) Structure of the mycobacterial ATP synthase Fo rotor ring in complex with the anti-TB drug bedaquiline. Sci Adv. doi:10.1126/sciadv.1500106
Runswick MJ, Bason JV, Montgomery MG, Robinson GC, Fearnley IM, Walker JE (2013) The affinity purification and characterization of ATP synthase complexes from mitochondria. Open Biol 3:120160. doi:10.1098/rsob.120160
Sakthivel S (2012) ATP-ase as a potential drug target for cancer, tumor growth and cellular functions. Int J Hum Genet 12:151–156
Salomon AR, Zhang Y, Seto H, Khosla C (2001) Structure-activity relationships within a family of selectively cytotoxic macrolide natural products. Org Lett 3:57–59
Sebald W, Wachter E, Tzagoloff A (1979) Identification of amino acid substitutions in the dicyclohexylcarbodiimide-binding subunit of the mitochondrial ATPase complex from oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem 100:599–607
Segala E, Sougakoff W, Nevejans-Chauffour A, Jarlier V, Petrella S (2012) New mutations in the mycobacterial ATP synthase: new insights into the binding of the diarylquinoline TMC207 to the ATP synthase C-ring structure. Antimicrob Agents Chemother 56:2326–2334. doi:10.1128/AAC.06154-11
Silverstein TP (2014) An exploration of how the thermodynamic efficiency of bioenergetic membrane systems varies with c-subunit stoichiometry of F1FO ATP synthases. J Bioenerg Biomembr 46:229–241. doi:10.1007/s10863-014-9547-y
Singh H, Watt NK, Garewal N, Pugarzenthan T (2013) Bedaquiline: a new weapon against MDR and XDR-TB. Int J Basic Clin Pharmacol 2:96–102. doi:10.5455/2319-2003.ijbcp20130301
Singh S, Kaur G, Mangia V, Gupta MK (2014) Quinoline and quinolones: promising scaffolds for future antimycobacterial agents. J Enzyme Inhib Med Chem. doi:10.3109/14756366.2014.930454
Steed PR, Fillingame RH (2008) Subunit a facilitates aqueous access to a membrane-embedded region of subunit c in Escherichia coli F1F0 ATP synthase. J Biol Chem 283:12365–12372
Stock D, Leslie AG, Walker JE (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286:1700–1705
Symersky J, Osowski D, Walters DE, Mueller DM (2012a) Oligomycin frames a common drug-binding site in the ATP synthase. Proc Natl Acad Sci USA 109:13961–13965. doi:10.1073/pnas.1207912109
Symersky J, Pagadala V, Osowski D, Krah A, Meier T, Faraldo-Gómez JD, Mueller DM (2012b) Structure of the c(10) ring of the yeast mitochondrial ATP synthase in the open conformation. Nat Struct Mol Biol 19(485–91):S1
Vollmar M, Schlieper D, Winn M, Büchner C, Groth G (2009) Structure of the c14 rotor ring of the proton translocating chloroplast ATP synthase. J Biol Chem 284:18228–18235. doi:10.1074/jbc.M109.006916
von Ballmoos C, Cook GM, Dimroth P (2008) Unique rotary ATP synthase and its biological diversity. Annu Rev Biophys 37:43–64. doi:10.1146/annurev.biophys.37.032807.130018
von Ballmoos C, Wiedenmann A, Dimroth P (2009) Essentials for ATP synthesis by F1FO ATP synthases. Annu Rev Biochem 78:649–672. doi:10.1146/annurev.biochem.78.081307
Vonck J, von Nidda TK, Meier T, Matthey U, Mills DJ, Kühlbrandt W, Dimroth P (2002) Molecular architecture of the undecameric rotor of a bacterial Na+-ATP synthase. J Mol Biol 321:307–316
Walker JE (2013) The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans 41:1–16. doi:10.1042/BST20110773
Watt IN, Montgomery MG, Runswick MJ, Leslie AG, Walker JE (2010) Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci USA 107:16823–16827. doi:10.1073/pnas.1011099107
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nesci, S., Trombetti, F., Ventrella, V. et al. The c-Ring of the F1FO-ATP Synthase: Facts and Perspectives. J Membrane Biol 249, 11–21 (2016). https://doi.org/10.1007/s00232-015-9860-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-015-9860-3