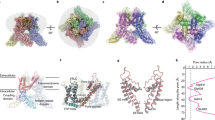
Abstract
Amphipathic polymers (amphipols), such as A8-35 and SApol, are a new tool for stabilizing integral membrane proteins in detergent-free conditions for structural and functional studies. Transient receptor potential (TRP) ion channels function as tetrameric protein complexes in a diverse range of cellular processes including sensory transduction. Mammalian TRP channels share ~20 % sequence similarity and are categorized into six subfamilies: TRPC (canonical), TRPV (vanilloid), TRPA (ankyrin), TRPM (melastatin), TRPP (polycystin), and TRPML (mucolipin). Due to the inherent difficulties in purifying eukaryotic membrane proteins, structural studies of TRP channels have been limited. Recently, A8-35 was essential in resolving the molecular architecture of the nociceptor TRPA1 and led to the determination of a high-resolution structure of the thermosensitive TRPV1 channel by cryo-EM. Newly developed maltose-neopentyl glycol (MNG) detergents have also proven to be useful in stabilizing TRP channels for structural analysis. In this review, we will discuss the impacts of amphipols and MNG detergents on structural studies of TRP channels by cryo-EM. We will compare how A8-35 and MNG detergents interact with the hydrophobic transmembrane domains of TRP channels. In addition, we will discuss what these cryo-EM studies reveal on the importance of screening different types of surfactants toward determining high-resolution structures of TRP channels.




Similar content being viewed by others
Abbreviations
- A8-35:
-
A poly(sodium acrylate)-based amphipol compromising 35 % of free carboxylates, 25 % of octyl chains, 40 % of isopropyl groups
- SApol:
-
Sulfonated amphipol
- DM:
-
Decyl-β-D-maltoside
- DDM:
-
Dodecyl-β-D-maltoside
- MNG:
-
Maltose-neopentyl glycol
- 2D:
-
2-Dimensional
- TM:
-
Transmembrane
- TRPA:
-
Transient receptor potential ankyrin
- TRPV:
-
Transient receptor potential vanilloid
- EM:
-
Electron microscopy
- SEC:
-
Size-exclusion chromatography
References
Althoff T, Mills DJ, Popot JL, Kuhlbrandt W (2011) Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J 30:4652–4664
Andrade EL, Meotti FC, Calixto JB (2012) TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther 133:189–204
Cao E, Liao M, Cheng Y, Julius D (2013) TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504:113–118
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436–441
Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Guittet E, Popot JL (2010) Solution NMR mapping of water-accessible residues in the transmembrane beta-barrel of OmpX. Eur Biophys J 39:623–630
Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D, Popot JL, Picot D, Fox BG, Guan L, Gether U, Byrne B, Kobilka B, Gellman SH (2010) Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods 7:1003–1008
Cvetkov TL, Huynh KW, Cohen MR, Moiseenkova-Bell VY (2011) Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J Biol Chem 286:38168–38176
Dahmane T, Giusti F, Catoire LJ, Popot JL (2011) Sulfonated amphipols: synthesis, properties, and applications. Biopolymers 95:811–823
Etzkorn, M, Zoonens, M, Catoire, LJ, Popot, JL, Hiller, S (2014). How amphipols embed membrane proteins: global solvent accessibility and interaction with a flexible protein terminus. J Membr Biol [Epub ahead of print]
Flotenmeyer M, Weiss H, Tribet C, Popot JL, Leonard K (2007) The use of amphipathic polymers for cryo electron microscopy of NADH: ubiquinone oxidoreductase (complex I). J Microsc 227:229–235
Fujiwara Y, Minor DL Jr (2008) X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J Mol Biol 383:854–870
Garavito RM, Ferguson-Miller S (2001) Detergents as tools in membrane biochemistry. J Biol Chem 276:32403–32406
Gohon Y, Popot JL (2003) Membrane protein-surfactant complexes. Curr Opin Coll Interface Sci 8:15–22
Gohon Y, Dahmane T, Ruigrok RW, Schuck P, Charvolin D, Rappaport F, Timmins P, Engelman DM, Tribet C, Popot JL, Ebel C (2008) Bacteriorhodopsin/amphipol complexes: structural and functional properties. Biophys J 94:3523–3537
Henderson R (2013) Structural biology: ion channel seen by electron microscopy. Nature 504:93–94
Huynh KW, Cohen MR, Chakrapani S, Holdaway HA, Stewart PL, Moiseenkova-Bell VY (2014) Structural insight into the assembly of TRPV channels. Structure 22:260–268
Inada H, Procko E, Sotomayor M, Gaudet R (2012) Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry 51:6195–6206
Jin X, Touhey J, Gaudet R (2006) Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem 281:25006–25010
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Kevany BM, Tsybovsky Y, Campuzano ID, Schnier PD, Engel A, Palczewski K (2013) Structural and functional analysis of the native peripherin-ROM1 complex isolated from photoreceptor cells. J Biol Chem 288:36272–36284
Lau SY, Procko E, Gaudet R (2012) Distinct properties of Ca2+-calmodulin binding to N- and C-terminal regulatory regions of the TRPV1 channel. J Gen Physiol 140:541–555
le Maire M, Champeil P, Moller JV (2000) Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta 1508:86–111
Liao M, Cao E, Julius D, Cheng Y (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504:107–112
Liao M, Cao E, Julius D, Cheng Y (2014) Single particle electron cryo-microscopy of a mammalian ion channel. Curr Opin Struct Biol 27C:1–7
Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R (2007) The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54:905–918
Moiseenkova-Bell VY, Wensel TG (2009) Hot on the trail of TRP channel structure. J Gen Physiol 133:239–244
Moiseenkova-Bell V, Wensel TG (2011) Functional and structural studies of TRP channels heterologously expressed in budding yeast. Adv Exp Med Biol 704:25–40
Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG (2008) Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci USA 105:7451–7455
Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R (2008) Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 47:2476–2484
Phelps CB, Wang RR, Choo SS, Gaudet R (2010) Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem 285:731–740
Popot JL, Berry EA, Charvolin D, Creuzenet C, Ebel C, Engelman DM, Flotenmeyer M, Giusti F, Gohon Y, Hong Q, Lakey JH, Leonard K, Shuman HA, Timmins P, Warschawski DE, Zito F, Zoonens M, Pucci B, Tribet C (2003) Amphipols: polymeric surfactants for membrane biology research. Cell Mol Life Sci 60:1559–1574
Popot JL, Althoff T, Bagnard D, Baneres JL, Bazzacco P, Billon-Denis E, Catoire LJ, Champeil P, Charvolin D, Cocco MJ, Cremel G, Dahmane T, de la Maza LM, Ebel C, Gabel F, Giusti F, Gohon Y, Goormaghtigh E, Guittet E, Kleinschmidt JH, Kuhlbrandt W, Le Bon C, Martinez KL, Picard M, Pucci B, Sachs JN, Tribet C, van Heijenoort C, Wien F, Zito F, Zoonens M (2011) Amphipols from A to Z. Annu Rev Biophys 40:379–408
Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK (2011a) Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature 469:175–180
Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK (2011b) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555
Ring AM, Manglik A, Kruse AC, Enos MD, Weis WI, Garcia KC, Kobilka BK (2013) Adrenaline-activated structure of β2-adrenoceptor stabilized by an engineered nanobody. Nature 502:575–579
Seddon AM, Curnow P, Booth PJ (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 1666:105–117
Shi DJ, Ye S, Cao X, Zhang R, Wang K (2013) Crystal structure of the N-terminal ankyrin repeat domain of TRPV3 reveals unique conformation of finger 3 loop critical for channel function. Protein Cell 4:942–950
Tribet C, Audebert R, Popot JL (1996) Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci USA 93:15047–15050
Tribet C, Diab C, Dahmane T, Zoonens M, Popot JL, Winnik FM (2009) Thermodynamic characterization of the exchange of detergents and amphipols at the surfaces of integral membrane proteins. Langmuir 25:12623–12634
Tsybovsky Y, Orban T, Molday RS, Taylor D, Palczewski K (2013) Molecular organization and ATP-induced conformational changes of ABCA4, the photoreceptor-specific ABC transporter. Structure 21:854–860
Vahedi-Faridi A, Jastrzebska B, Palczewski K, Engel A (2013) 3D imaging and quantitative analysis of small solubilized membrane proteins and their complexes by transmission electron microscopy. Microscopy (Oxf) 62:95–107
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Westfield GH, Rasmussen SG, Su M, Dutta S, DeVree BT, Chung KY, Calinski D, Velez-Ruiz G, Oleskie AN, Pardon E, Chae PS, Liu T, Li S, Woods VL Jr, Steyaert J, Kobilka BK, Sunahara RK, Skiniotis G (2011) Structural flexibility of the G alpha s alpha-helical domain in the beta2-adrenoceptor Gs complex. Proc Natl Acad Sci USA 108:16086–16091
Wilkens S (2000) F1F0-ATP synthase-stalking mind and imagination. J Bioenerg Biomembr 32:333–339
Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J (2001) Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell 7:1047–1057
Zhang C, Srinivasan Y, Arlow DH, Fung JJ, Palmer D, Zheng Y, Green HF, Pandey A, Dror RO, Shaw DE, Weis WI, Coughlin SR, Kobilka BK (2012) High-resolution crystal structure of human protease-activated receptor 1. Nature 492:387–392
Zoonens M, Popot JL (2014). Amphipols for each season. J Membrane Biol. doi:10.1007/s00232-014-9666-8
Acknowledgments
We would like to thank Teresa Cvetkov for her contribution to this project. We are also very grateful to Jean-Luc Popot for providing us with amphipols and helpful discussions. This work was supported by the American Heart Association (NCRP Scientist Development Grant 11SDG5280029), the American Lung Association Biomedical Research Grant (RG-166985-N), and the National Institute of Health Grant (NIGMS 1R01GM103899-01A1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huynh, K.W., Cohen, M.R. & Moiseenkova-Bell, V.Y. Application of Amphipols for Structure–Functional Analysis of TRP Channels. J Membrane Biol 247, 843–851 (2014). https://doi.org/10.1007/s00232-014-9684-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-014-9684-6