Abstract
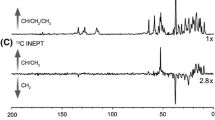
The atomic structure of OmpX, the smallest member of the bacterial outer membrane protein family, has been previously established by X-ray crystallography and NMR spectroscopy. In apparent conflict with electrophysiological studies, the lumen of its transmembrane β-barrel appears too tightly packed with amino acid side chains to let any solute flow through. In the present study, high-resolution solution NMR spectra were obtained of OmpX kept water-soluble by either amphipol A8-35 or the detergent dihexanoylphosphatidylcholine. Hydrogen/deuterium exchange measurements performed after prolonged equilibration show that, whatever the surfactant used, some of the amide protons of the membrane-spanning region exchange much more readily than others, which likely reflects the dynamics of the barrel.







Similar content being viewed by others
References
Ahn VE, Lo EI, Engel CK, Chen L, Hwang PM, Kay LE, Bishop RE, Privé GG (2004) A hydrocarbon ruler measures palmitate in the enzymatic acylation of endotoxin. EMBO J 23:2931–2941
Arnold T, Poynor M, Nussberger S, Lupas AN, Linke D (2007) Gene duplication of the eight-stranded β-barrel OmpX produces a functional pore: a scenario for the evolution of transmembrane β-barrel. J Mol Biol 366:1174–1184
Baldermann C, Lupas A, Lubieniecki J, Engelhardt H (1998) The regulated outer membrane protein Omp21 from Comamonas acidovorans is identified as a member of a new family of eight-stranded beta-sheet proteins by its sequence and properties. J Bacteriol 180:3741–3749
Böckmann RA, Caflisch A (2005) Spontaneous formation of detergent micelles around the outer membrane protein X. Biophys J 88:3191–3204
Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Popot J-L, Guittet É (2009) Inter- and intramolecular contacts in a membrane protein/surfactant complex observed by heteronuclear dipole-to-dipole cross-relaxation. J Magn Reson 197:91–95
Cox K, Bond PJ, Grottesi A, Baaden M, Sansom MS (2008) Outer membrane proteins: comparing X-ray and NMR structures by MD simulations in lipid bilayers. Eur Biophys J 37:131–141
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Diab C, Tribet C, Gohon Y, Popot J-L, Winnik FM (2007) Complexation of integral membrane proteins by phosphorylcholine-based amphipols. Biochim Biophys Acta 1768:2737–2747
Dupont M, Dé E, Chollet R, Chevalier J, Pagès J-M (2004) Enterobacter aerogenes OmpX, a cation-selective channel mar- and osmo-regulated. FEBS Lett 569:27–30
Fernández C, Adeishvili K, Wüthrich K (2001) Transverse relaxation-optimized NMR spectroscopy with the outer membrane protein OmpX in dihexanoyl phosphatidylcholine micelles. Proc Natl Acad Sci USA 98:2358–2363
Fernández C, Hilty C, Wider G, Günter P, Wüthrich K (2004) NMR structure of the integral membrane protein OmpX. J Mol Biol 336:1211–1221
Gohon Y, Pavlov G, Timmins P, Tribet C, Popot, J-L, Ebel C (2004) Partial specific volume and solvent interactions of amphipol A8-35. Anal Biochem 334:318–334
Gohon Y, Giusti F, Prata C, Charvolin D, Timmins P, Ebel C, Tribet C, Popot, J-L (2006) Well-defined nanoparticles formed by hydrophobic assembly of a short and polydisperse random terpolymer, amphipol A8-35. Langmuir 22:1281–1290
Gohon Y, Dahmane T, Ruigrok R, Schuck P, Charvolin D, Rappaport F, Timmins P, Engelman DM, Tribet C, Popot J-L, Ebel C (2008) Bacteriorhodopsin/amphipol complexes: structural and functional properties. Biophys J 94:3523–3537
Hearn EM, Patel DR, Lepore BW, Indic M, van den Berg B (2009) Transmembrane passage of hydrophobic compounds through a protein channel wall. Nature 458:367–370
Hong H, Szabo G, Tamm LK (2006a) Electrostatic couplings in OmpA ion-channel gating suggest a mechanism for pore opening. Nat Chem Biol 2:627–635
Hong H, Patel DR, Tamm LK, van den Berg B (2006b) The outer membrane protein OmpW forms an eight-stranded beta-barrel with a hydrophobic channel. J Biol Chem 281:7568–7577
Hwang PM, Choy WY, Lo EI, Chen L, Forman-Kay JD, Raetz CR, Privé GG, Bishop RE, Kay LE (2002) Solution structure and dynamics of the outer membrane enzyme PagP by NMR. Proc Natl Acad Sci USA 99:13560–13565
Johnson BA, Blevins RA (1994) NMRView: a computer program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614
Lee D, Hilty C, Wider G, Wüthrich K (2006) Effective rotational correlation times of proteins from NMR relaxation interference. J Magn Reson 178:72–76
Pervushin K, Riek R, Wider G, Wüthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole–dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA 94:12366–12371
Popot J-L, Berry EA, Charvolin D, Creuzenet C, Ebel C, Engelman DM, Flötenmeyer M, Giusti F, Gohon Y, Hervé P, Hong Q, Lakey JH, Leonard K, Shuman HA, Timmins P, Warschawski DE, Zito F, Zoonens M, Pucci B, Tribet C (2003) Amphipols: polymeric surfactants for membrane biology research. Cell Mol Life Sci 60:1559–1574
Sharma KS, Durand G, Giusti F, Olivier B, Fabiano AS, Bazzacco P, Dahmane T, Ebel C, Popot J-L, Pucci B (2008) Glucose-based amphiphilic telomers designed to keep membrane proteins soluble in aqueous solutions: synthesis and physical–chemical characterization. Langmuir 24:13581–13590
Tribet C, Audebert R, Popot J-L (1996) Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci USA 93:15047–15050
Vogt J, Schulz GE (1999) The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure 7:1301–1309
Zoonens M, Catoire LJ, Giusti F, Popot J-L (2005) NMR study of a membrane protein in detergent-free aqueous solution. Proc Natl Acad Sci USA 102:8893–8898
Acknowledgments
This work was funded by the CNRS, Paris-7 University, and financial support to J.-L.P. by the HFSP Organization (grant RG00223/2000-M) and the Fondation Rothschild.
Author information
Authors and Affiliations
Corresponding author
Additional information
The more you see: Spectroscopy in molecular biophysics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Catoire, L.J., Zoonens, M., van Heijenoort, C. et al. Solution NMR mapping of water-accessible residues in the transmembrane β-barrel of OmpX. Eur Biophys J 39, 623–630 (2010). https://doi.org/10.1007/s00249-009-0513-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-009-0513-2