Abstract
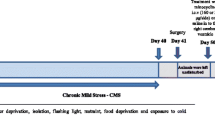
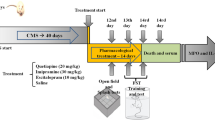
The chronic mild stress (CMS) protocol is widely used to evoke depression-like behaviors in the laboratory. Some animals exposed to CMS are resistant to the development of anhedonia, whereas the remaining are responsive, CMS-resilient and CMS-sensitive, respectively. The aim of this study was to examine the effects of chronic stress on oxidative parameters in the rat brain. The consumption of sweet food, protein and lipid oxidation levels and superoxide dismutase and catalase activities in the rat hippocampus, cortex and cerebellum were assessed. We found a significant increase in protein peroxidation (hippocampus and cortex), a significant increase in catalase activity (cortex, hippocampus and cerebellum) and a decrease in superoxide dismutase activity (cortex, hippocampus and cerebellum) in the CMS-sensitive group compared to the CMS-resilient group and normal controls as well as an increase in lipid peroxidation (cerebellum) in the CMS-sensitive and CMS-resilient groups compared to normal controls. However, there was no significant difference in protein peroxidation (cerebellum) and lipid peroxidation (cortex and hippocampus) among the three groups. In conclusion, our results indicate that the segregation into CMS-sensitive and -resilient groups based on sucrose intake is paralleled by significant differences in oxidative parameters. CMS induces oxidative damage and alterations in the activity of antioxidants which may lead to increased oxidative damage, irrespective of the anhedonia-like status of the stressed animals.




Similar content being viewed by others

References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z (2005) Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res 161:45–59
Bergstrom A, Jayatissa MN, Thykjaer T, Wiborg O (2007) Molecular pathways associated with stress resilience and drug resistance in the chronic mild stress rat model of depression: a gene expression study. J Mol Neurosci 33:201–215
Bergstrom A, Jayatissa MN, Mork A, Wiborg O (2008) Stress sensitivity and resilience in the chronic mild stress rat model of depression: an in situ hybridization study. Brain Res 1196:41–52
Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O (2001) Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 64:43–51
Bisgaard CF, Jayatissa MN, Enghild JJ, Sanchez C, Artemychyn R, Wiborg O (2007) Proteomic investigation of the ventral rat hippocampus links DRP-2 to escitalopram treatment resistance and SNAP to stress resilience in the chronic mild stress model of depression. J Mol Neurosci 32:132–144
Carvalho F, Fernandes E, Remiao F, Gomes-Da-Silva J, Tavares MA, Bastos MD (2001) Adaptative response of antioxidant enzymes in different areas of rat brain after repeated d-amphetamine administration. Addict Biol 6:213–221
Darley-Usmar VM, Hogg N, O’Leary VJ, Wilson MT, Moncada S (1992) The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun 17:9–20
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Floyd RA (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med 222:236–245
Fontella FU, Siqueira IR, Vasconcellos AP, Tabajara AS, Netto CA, Dalmaz C (2005) Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res 30:105–111
Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C (2003) Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int 42:107–114
Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O (2008) Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem 89:560–566
Henningsen K, Andreasen JT, Bouzinova EV, Jayatissa MN, Jensen MS, Redrobe JP, Wiborg O (2009) Cognitive deficits in the rat chronic mild stress model for depression: relation to anhedonia-like responses. Behav Brain Res 198:136–141
Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O (2006) Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 31:2395–2404
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Liu J, Wang X, Shigenaga MK, Yeo HC, Mori A, Ames BN (1996) Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J 10:1532–1538
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lucca G, Comim CM, Valvassori SS, Reus GZ, Vuolo F, Petronilho F, Dal-Pizzol F, Gavioli EC, Quevedo J (2009a) Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 54:358–362
Lucca G, Comim CM, Valvassori SS, Reus GZ, Vuolo F, Petronilho F, Gavioli EC, Dal-Pizzol F, Quevedo J (2009b) Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res 43:864–869
Moreau JL, Scherschlicht R, Jenck F, Martin JR (1995) Chronic mild stress-induced anhedonia model of depression: sleep abnormalities and curative effects of electroshock treatment. Behav Pharmacol 6:682–687
Papp M, Willner P, Muscat R (1991) An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 104:255–259
Peet M, Murphy B, Shay J, Horrobin D (1998) Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 43:315–319
Sanchez C, Gruca P, Papp M (2003) R-Citalopram counteracts the antidepressant-like effect of escitalopram in a rat chronic mild stress model. Behav Pharmacol 14:465–470
Sarandol A, Kirli S, Akkaya C, Altin A, Demirci M, Sarandol E (2007) Oxidative–antioxidative systems and their relation with serum S100 B levels in patients with schizophrenia: effects of short term antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry 31:1164–1169
Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P (2004) Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29:2007–2017
Willner P (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134:319–329
Willner P, Muscat R, Papp M (1992) Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16:525–534
Acknowledgments
This work was supported by the Institute of Functional Brain Disorders of PLA.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Wang, H. Wu and X. Jing have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, C., Wu, Hm., Jing, Xr. et al. Oxidative Parameters in the Rat Brain of Chronic Mild Stress Model for Depression: Relation to Anhedonia-Like Responses. J Membrane Biol 245, 675–681 (2012). https://doi.org/10.1007/s00232-012-9436-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-012-9436-4