Abstract
Transient lateral microdomains or lipid rafts play important roles in many physiological membrane-mediated cell processes. Detergent-resistant membranes (DRMs) are good models for the study of lipid rafts. Here we report that DRMs can be obtained by treating human erythrocytes with the nonionic detergents Triton X-100 or octaethylene glycol monododecyl ether (C12E8) at 37°C, and by treatment at 4°C of cholesterol-depleted erythrocytes. Electron paramagnetic resonance with spin labels inserted at different membrane depths (5- and 16-doxyl stearic acids, 5-SASL and 16-SASL) were used to measure the order parameter (S) of the cell membranes and DRMs. We previously reported significantly higher S values in DRMs with respect to intact erythrocyte membranes. Here we show that higher S values were still measurable in DRMs prepared from intact erythrocytes at 37°C, or from cholesterol-depleted cells at 4°C, for both detergents. For 5-SASL only, increased S values were measured in 4°C DRMs obtained from cholesterol-depleted versus intact erythrocytes. Flotillin-2, a protein marker of lipid rafts, was found in DRMs from intact cells in trace amounts but it was sensitively increased in C12E8 DRMs prepared at 4°C from cholesterol-depleted erythrocytes, while the membrane-skeletal proteins spectrin and actin were excluded from both Triton X-100 and C12E8 DRMs. However, contrary to the 4°C treatment results, flotillin-2 and stomatin were not resistant to Triton X-100 and C12E8 treatment at physiological temperature. The role of cholesterol in DRMs formation is discussed and the results presented provide further support for the use of C12E8 to the study of DRMs.




Similar content being viewed by others
Notes
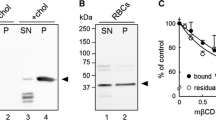
Free cholesterol in human red blood cells is 3.15 μmol/ml cells, while total lipid phosphorus is 3.90 μmol/ml cells (Dodge and Phillips 1967). Cholesterol is therefore present in the cell membrane at approximately 45 mol% with respect to total lipids. In DRMs from normal cells, we have 30% of the cell cholesterol, corresponding to approximately 0.945 μmol (per milliliter of cells). If we assume that the sphingolipid-enriched, detergent-resistant phase early described in the literature (before the notion of lipid rafts was introduced) contained what we now call DRMs (and can be released from the membrane skeleton only by treatment with carbonate), then the sphingomyelin in the detergent-resistant portion of the erythrocyte, obtained with Triton X-100 at concentrations comparable to those we used, corresponds approximately to 70–80% of the cell sphingomyelin (Yu et al. 1973; Sheetz 1979). Because the latter corresponds to 25 mol% of total cell lipid phosphorus (Dodge and Phillips 1967), it amounts to 1.0 μmol (per milliliter of cells), and its content in the DRMs should be approximately 0.7–0.8 μmol. Therefore, the cholesterol percentage with respect to the total of DRM lipids (i.e., sphingomyelin plus cholesterol, with the reasonable approximation that the other phospholipids are present in DRMs in much lower amount) is 54–57%.
References
An X, Guo X, Liu S, Lux SE, Baines A, Gratzer W, Mohandas N (2005) Identification and functional characterization of protein 4.1R and actin-binding sites in erythrocyte beta spectrin: regulation of the interactions by phosphatidylinositol-4,5-bisphosphate. Biochemistry 44:10681–10688
An X, Zhang X, Debnath G, Baines AJ, Mohandas N (2006) Phosphatidylinositol-4,5-biphosphate (PIP2) differentially regulates the interaction of human erythrocyte protein 4.1 (4.1R) with membrane proteins. Biochemistry 45:5725–5732
Anderson RG, Jacobson K (2002) A role for lipid shells in targeting proteins to caveolae, rafts and other lipid domains. Science 296:1821–1825
Beutler E, West C, Blume KG (1976) The removal of leukocytes and platelets from whole blood. J Lab Clin Med 88:328–333
Brown DA (2006) Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 21:430–439
Brown DA (2007) Analysis of raft affinity of membrane proteins by detergent-insolubility. In: McIntosh TJ (ed) Methods in molecular biology. Lipid rafts. Humana Press, Totowa, NJ, pp 1–7
Brown DA, London E (1998a) Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14:111–136
Brown DA, London E (1998b) Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164:103–114
Brown DA, London E (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275:17221–17224
Brown DA, Rose JK (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533–544
Cassera MB, Silber AM, Gennaro AM (2002) Differential effects of cholesterol on acyl chain order in erythrocyte membranes as a function of depth from the surface. An electron paramagnetic resonance (EPR) spin label study. Biophys Chem 99:117–127
Ciana A, Balduini C, Minetti G (2005) Detergent-resistant membranes in human erythrocytes and their connection to the membrane-skeleton. J Biosci 30:317–328
Crepaldi Domingues C, Ciana A, Buttafava A, Balduini C, de Paula E, Minetti G (2009) Resistance of human erythrocyte membranes to Triton X-100 and C12E8. J Membr Biol 227:39–48
Delaunay JL, Breton M, Godine JW, Trugnan G, Maurice M (2007) Differential detergent resistance of the apical and basolateral NPPases: relationship with polarized targeting. J Cell Sci 120:1009–1016
Diakowski W, Ozimek Ł, Bielska E, Bem S, Langner M, Sikorski AF (2006) Cholesterol affects spectrin-phospholipid interactions in a manner different from changes resulting from alterations in membrane fluidity due to fatty acyl chain composition. Biochim Biophys Acta 1758:4–12
Dietrich C, Volovyk ZN, Levi M, Thompson NL, Jacobson K (2001) Partitioning of Thy-1, GM1 and crosslinked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc Natl Acad Sci USA 98:10642–10647
Dietrich C, Yang B, Fujiwara T, Kusumi A, Jacobson K (2002) Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys J 82:274–284
Dodge JT, Phillips GB (1967) Composition of phospholipids and of phospholipid fatty acids and aldehydes in human red cells. J Lipid Res 8:667–675
Fraceto LF, Pinto Lde M, Franzoni L, Braga AA, Spisni A, Schreier S, de Paula E (2002) Spectroscopic evidence for a preferential location of lidocaine inside phospholipid bilayers. Biophys Chem 99:229–243
Fragoso R, Ren D, Zhang X, Su MW, Burakoff SJ, Jin YJ (2003) Lipid raft distribution of CD4 depends on its palmitoylation and association with Lck, and evidence for CD4-induced lipid raft aggregation as an additional mechanism to enhanced CD3 signaling. J Immunol 170:913–921
Godici PE, Landsberger FR (1974) Dynamics structure of lipid-membranes: C13 nuclear magnetic resonance study using spin labels. Biochemistry 13:362–368
Hägerstrand H, Isomaa B (1989) Vesiculation induced by amphiphiles in erythrocytes. Biochim Biophys Acta 982:179–186
Hägerstrand H, Isomaa B (1992) Morphological characterization of exovesicles and endovesicles released from human erythrocytes after treatment with amphiphiles. Biochim Biophys Acta 1109:117–126
Hägerstrand H, Kralj-Iglic V, Fošnarič M, Bobrowska-Hägerstrand M, Wróbel A, Mrówczyńska L, Söderstöm T, Iglič A (2004) Endovesicle formation and membrane perturbation induced by polyoxyethyleneglycolalkylethers in human erythrocytes. Biochim Biophys Acta 1665:191–200
Hubbel WL, McConnel HM (1971) Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc 93:314–326
Ilangumaran S, Hoessli DC (1998) Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J 335:433–440
Ilangumaran S, Arni S, van Echten-Decker G, Borisch B, Hoessli DC (1999) Microdomain-dependent regulation of Lck and Fyn protein-tyrosine kinases in T lymphocyte plasma membranes. Mol Biol Cell 10:891–905
Kessel A, Ben-Tal N, May S (2001) Interactions of cholesterol with lipid bilayers: the preferred configuration and fluctuations. Biophys J 81:643–658
Koumanov KS, Tessier C, Momchilova AB, Rainteau D, Wolf C, Quinn PJ (2005) Comparative lipid analysis and structures of detergent-resistant membrane raft fractions isolated from human and ruminant erythrocytes. Arch Biochem Biophys 434:150–158
Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M (2003) Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci USA 100:13964–13969
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680–685
Lichtenberg D, Goni FM, Heerklotz H (2005) Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci 30:430–436
London E, Brown DA (2000) Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim Biophys Acta 1508:182–195
Matkó J, Bodnár A, Vereb G, Bene L, Vámosi G, Szentesi G, Szöllösi J, Gáspár R, Horejsi V, Waldmann TA, Damjanovich S (2002) GPI-microdomains (membrane rafts) and signaling of the multi-chain interleukin-2 receptor in human lymphoma/leukemia T cell lines. Eur J Biochem 269:1199–2008
Murphy SC, Samuel BU, Harrison T, Speicher KD, Speicher DW, Reid ME, Prohaska R, Low PS, Tanner MJ, Mohandas N, Haldar K (2004) Erythrocyte detergent-resistant membrane proteins: their characterization and selective uptake during malaria infection. Blood 103:1920–1928
Neumann-Giesen C, Falkenbach B, Beicht P, Claasen S, Lüers G, Stuermer CA, Herzog V, Tikkanen R (2004) Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem J 378:509–518
Nishijo J, Moriyama S, Shiota S (2003) Interactions of cholesterol with cyclodextrins in aqueous solution. Chem Pharm Bull 51:1253–1257
Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J (1989) Differential effects of α-, β- and γ-cyclodextrins on human erythrocytes. Eur J Biochem 186:17–22
Pike LJ (2003) Lipid rafts: bringing order to chaos. J Lipid Res 44:655–667
Pike LJ (2004) Lipid rafts: heterogeneity on the high seas. Biochem J 378:281–292
Pike LJ (2006) Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipid Res 47:1597–1598
Preté PSC, Gomes K, Malheiros SVP, Meirelles NC, de Paula E (2002) Solubilization of human erythrocyte membranes by non-ionic surfactants of the polyoxyethylene alkyl ethers series. Biophys Chem 97:45–54
Radhakrishnan A, Li XM, Brown RE, McConnell HM (2001) Stoichiometry of cholesterol–sphingomyelin condensed complexes in monolayers. Biochim Biophys Acta 1511:1–6
Rietveld A, Simons K (1998) The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim Biophys Acta 1376:467–479
Rivas MG, Gennaro AM (2003) Detergent resistant domains in erythrocyte membranes survive after cell cholesterol depletion: an EPR spin label study. Chem Phys Lipids 122:165–169
Rodi PM, Trucco VM, Gennaro AM (2008) Factors determining detergent resistance of erythrocyte membranes. Biophys Chem 135:14–18
Rose HG, Oklander M (1965) Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res 6:428–431
Salzer U, Prohaska R (2001) Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 97:1141–1143
Samuel BU, Mohandas N, Harrison T, McManus H, Rosse W, Reid M, Haldar K (2001) The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J Biol Chem 276:29319–29329
Schreier S, Polnaszek CF, Smith ICP (1978) Spin labels in membranes. Biochim Biophys Acta 515:375–436
Schroeder R, London E, Brown DA (1994) Interactions between saturated acyl chains confer detergent resistance on lipids and GPI-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci USA 91:12130–12134
Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K (2003) Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 100:5795–5800
Sheetz MP (1979) Integral membrane protein interaction with Triton cytoskeletons of erythrocytes. Biochim Biophys Acta 557:122–134
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Simons K, Vaz WL (2004) Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struc 33:269–295
Vazquez MJ, Rivas MG, Gennaro AM (2002) Modification of cholesterol content in human red cell membranes by using methyl-β-cyclodextrin: time evolution and cell shape changes. Rev FABICIB 6:121–127
Warren RC (1987) Physics and architecture of cell membranes. Adam-Hilger, Bristol
Wilkinson DK, Turner EJ, Parkin ET, Garner AE, Harrison PJ, Crawford M, Stewart GW, Hooper NM (2007) Membrane raft actin deficiency and altered Ca2+-induced vesiculation in stomatin-deficient overhydrated hereditary stomatocytosis. Biochim Biophys Acta 1778:125–132
Xu X, London E (2000) The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 39:843–849
Yu J, Fischman DA, Steck TL (1973) Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct 1:233–248
Acknowledgments
This work was supported by PRIN funds of the “Ministero dell’Università e della Ricerca,” Italy, and FAPESP (Proc. 09/00904-1), Brazil. CCD acknowledges the fellowships from CAPES (Proc. 3597/06-7) and CNPq (Proc. 141618/2005-1), Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. C. Domingues and A. Ciana have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Domingues, C.C., Ciana, A., Buttafava, A. et al. Effect of Cholesterol Depletion and Temperature on the Isolation of Detergent-Resistant Membranes from Human Erythrocytes. J Membrane Biol 234, 195–205 (2010). https://doi.org/10.1007/s00232-010-9246-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-010-9246-5