Abstract
Efficient heat transfer is essential for the economically sustainable operation of heat exchangers. Therefore, the internal flow is influenced systematically in various ways, for example by introducing macrostructures on the pipe surface. Since these measures may negatively affect the cleanability of the heat exchanger, it is necessary to investigate not only the increase in heat transfer, but also their impact on cleaning processes. For this purpose, the cleaning of sour milk in dimple-structured pipes is investigated experimentally. Both macroscopic and microbial cleaning tests are conducted to assess the influence of the surface’s macrostructure on cleanability. Two geometry variations of dimple-structured pipes are investigated and compared to a straight pipe Although fouling is enhanced by the dimple structures, a higher macroscopic cleaning rate can be achieved with the optimized dimple. Moreover, the residual microbial contamination decreases significantly due to the introduction of dimples. All in all, the cleaning experiments confirm the positive influence of the dimple structures on cleanability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Additive manufacturing or 3D printing bears a lot of potential, which is why it is widespread and used in aerospace, automotive, biomedical, architecture and electronics industries [1,2,3,4]. It offers the advantages of design freedom, great adaptability, high material utilization, rapid prototyping, and the capacity to form complex components without weld seams or the like [5].
Additive manufacturing also opens up new possibilities for the design and development of heat exchangers [6]. Efficient heat transfer is essential for their economically sustainable operation. Therefore, the internal flow is influenced systematically in various ways, for example by turbulators, fins or swirl flow devices [7]. Additive manufacturing technologies enable engineers to develop geometric designs with a high internal and external complexity in order to improve heat transfer whilst minimizing pressure drop [8,9,10].
Heat exchangers used in food production must meet special requirements, as the food industry places particularly high demands on the materials used, especially for surfaces that are in contact with the product. Austenitic stainless steel is normally used for the components of process lines because of its good mechanical properties as well as corrosion resistance [11]. Selective laser melting (SLM) is a promising technology that can be used to additively manufacture grade 316L stainless steel. Owing to the nature of the process, components manufactured with SLM exhibit a high surface roughness. This contradicts the hygienic design principles, which recommend a small profile roughness of \({R}_{a}<0.8\, \mathrm{\mu m}\) [12]. Accordingly, additively manufactured components are inferior to conventionally manufactured components because the rough surfaces extremely promote fouling and make cleaning more difficult. This limits their use and prevents their application in the food industry [13]. However, the surface topography correlates with the microscopic and macroscopic properties and both determine the surface properties relevant to cleaning, e.g. surface wetting [14]. Higher roughness on product contact surfaces is acceptable if cleanability has been verified.
One way to address the inferior microstructure of the surface is to influence its macrostructure to improve surface-normal transport processes [15]. Among the variety of possible structures, concave-shaped dimples have shown promise in improving heat transfer while minimizing pressure drop increase [16, 17].
While there was a lot of research concerning the heat transfer augmentation of macrostructures, there is only limited literature regarding their effect on cleaning. Murcek et al. [18]. have shown that the use of surface modifications can also contribute to an improvement in a component's cleanability. They observed an increase in the cleaning rate of up to 60 % for selected structures and food contaminants. Recent studies also address the potential of dimpled surfaces to reduce fouling [19,20,21,22]. Experimental and computational studies of dimpled surfaces in a channel flow indicated a lower tendency for particulate fouling to occur in and around the spherical dimple compared to a square cavity. This holds true for a package of dimples in a staggered arrangement as well.
Köhler et al. [23]. outlined how to evaluate the cleanability of machinery by means of numerical simulations. Their approach is based on the four prototypical cleaning mechanisms for film-like soils, i.e. diffusive dissolution, cohesive separation, viscous shifting and adhesive detachment [24, 25]. Köhler et al. [23]. propose specific modeling approaches for each of the cleaning mechanisms that can be used to predict cleanability. On this basis, Joppa et al. [26]. derived an approach to evaluate and optimize the cleanability of a dimple-structured pipe surface utilizing computational fluid dynamics (CFD) simulations.
In the present publication, the experimental validation of this procedure is provided. The macrostructures determined by CFD simulations and integrated into pipe geometries by metallic 3D printing will be subjected to cleaning experiments. By measuring the macroscopic and microbial cleaning behavior, the effects of the different macrostructures on cleanability will be identified.
2 Experimental methods
2.1 Investigated surfaces
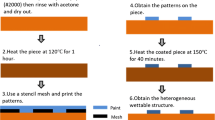
Test sections made of AISI 316L stainless steel with an inner diameter of \(D=25 \,\mathrm{mm}\) were additively manufactured using the SLM process. The components were designed and manufactured with a parting plane. This made it possible to perform macroscopic cleaning experiments by connecting the individual halves with a counterpart in which a UV light-transmissive acrylic glass was integrated, see Fig. 1. The surface of the 3D-printed, metallic components was not post-processed so that their roughness was \({R}_{a}=4.3\, \mathrm{\mu m}\) on average.
Figure 2 shows images of the investigated surfaces. The straight pipe as built by the 3D printing process serves as a reference for two promising macrostructures that were identified through 3D-CFD simulations by Joppa et al. [26]. The basic shape of both macrostructures is a spherical dimple which is created by the intersection of a sphere with the pipe surface. The dimple structures are arranged in a staggered layout with an axial distance of 10 mm between two consecutive dimple rows. 12 dimples are placed around the circumference of the pipe leading to an angular interspace of 30°. The basic dimple has a width and depth of 3.6 mm and 0.6 mm, respectively. With the help of CFD simulations, its shape was optimized by Joppa et al. [26]. This optimized dimple has a width of 6.25 mm and a depth of 1.125 mm which leads to partial intersection with the adjacent dimple structures. Further details about the geometric dimensions can be found in [26].
2.2 Macroscopic cleaning experiments
In the cleaning experiments, sour milk was used as typical food-based model soil [27,28,29]. This was prepared from 1 L of low-fat milk with 0,1 g of a mesophilic mixed culture (Danisco Choozit MM 100 LYO 25 DCU). Afterward, the mixture was incubated at \(T=30\,^\circ \mathrm{C}\) for 18 h and homogenized before applying the soil to the test sections.
For soiling, the metallic halves of the test sections were joined together, completely filled with the homogeneous soil and sealed. Subsequently, the test sections including the soil were mechanically stressed for 60 s with a vortex mixer. Afterward, they were dried at a temperature of 30 °C and 50 % relative humidity for 24 h. During the drying process, the test sections were stored in an upright position so that the soil could run out at the bottom. The initial surface soil coverage \({m}_{s,0}\) was determined by differential weighing of the test sections before soiling and after drying.
The basic setup of the cleaning experiments is shown in Fig. 3. The cleaning fluid, a mild, alkaline detergent solution according to Guideline 2 of the European Hygienic Engineering Design Group (EHEDG) [29] was stored in a large tank and heated up to an operating temperature of \(T=63\,^\circ \mathrm{C}\). An EBARA 2CDXHS 70/12 centrifugal pump continuously circulated it through the experimental facility. For the macroscopic cleaning experiments, the metallic, soiled part and the acrylic glass part of the test sections were joined together so that optical accessibility was ensured. Afterward, the test sections were mounted horizontally in the test rig and cleaned using the parameters specified in the previous paragraph.
The cleaning process was monitored by a camera system that detects the fluorescence of the soil induced by UVA light. A Baumer VCXU-51C industrial camera took images at a rate of 1 frame per second. In these images, the soil layer appears bright in comparison to the dark metallic surface of the test section. The local fluorescence intensity, and therefore also the local brightness in the image, correlates with the thickness of the soil layer [30,31,32]. Hence, the average greyscale value in a region of interest can be used as a parameter for the relative amount of residual soil. The development of the greyscale value over time is representative of the cleaning progress [33]. In preliminary tests, it was found that dried sour milk is comparatively easy to remove. Therefore, the mean velocity in the test section, i.e., in the pipe, was reduced from \(u=\mathrm{1,5} \,\mathrm{m}/\mathrm{s}\) in the EHEDG test to \(u=\mathrm{0,34}\, \mathrm{m}/\mathrm{s}\) in order to be able to detect relevant differences between the different surface structures at the given frame rate. Each cleaning experiment was performed at least four times to ensure adequate statistical confidence.
2.3 Microbial cleaning experiments
In addition to the macroscopic cleaning process, the microbial cleaning behavior of the different surface structures was assessed. For this purpose, a spore suspension of Geobacillus stearothermophilus was added to the sour milk soil with a concentration of \({10}^{5}\) colony forming units (CFU) per milliliter.
The microbial cleaning experiments used the same cleaning fluid and were carried out in accordance with EHEDG Guideline 2 [29]. A slightly modified cleaning protocol was applied here: rinsing with cold water for 1 min, cleaning with a mild, alkaline detergent solution at a temperature of \(T=63\,^\circ \mathrm{C}\) and an average flow velocity of \(u=\mathrm{1,5}\, \mathrm{m}/\mathrm{s}\) for 18 min and, finally, rinsing with cold water for 1 min.
To evaluate microbial contamination, the test sections were removed from the test rig, filled with a special purple growth medium (Shapton & Hinds Agar) [29] and incubated at a temperature of 58 °C for 24 h. Subsequently, the agar was removed and analyzed. The spores of G. stearothermophilus react with the pH indicator (bromocresol purple) in the agar leading to a yellow discoloration of not successfully cleaned areas. A quantitative assessment of the residual microbial contamination was carried out by determining the percentage of yellow discolored areas. For this purpose, images of the incubated agar were taken, the white areas separated, and the yellow areas determined automatically using a MATLAB program, as indicated in Fig. 4.
3 Results and discussion
3.1 Fouling behavior
At first, the influence of the surface structures on the fouling behavior is presented. Since the structures lead to different surface areas, the measured soil mass was normalized with the surface area. Figure 5 shows the average initial surface soil coverage dependent on the surface structure. The results indicate that the structured surfaces promote fouling compared to the straight pipe. While the surface soil coverage of the pipe with the basic dimples is only about 10% higher than that of the straight pipe, it increases by 70% for the optimized dimples.
A one-way analysis of variance (ANOVA) was conducted to compare the effect of the surface design on initial surface soil coverage. There was a statistically significant difference in mean initial surface soil coverage at the p < 0.05 level between at least two designs [F(2, 9) = 25.74, p = 0.0002]. Post-hoc comparisons using Tukey's range test [34] indicated that the mean value of initial surface soil coverage of the optimized dimple shape was significantly higher than that of the straight reference pipe (p = 0.0002, 95% confidence interval (C.I.) = [12.43, 30.42]) and that of the basic dimple (p = 0.0008, 95% C.I. = [9.25, 27.24]).
This is due to the fact, that the cavities are significantly larger for the optimized dimple. The soil accumulates primarily on the sharp edges of the dimple, which were facing downward during drying, as can be seen in the images after one second in Fig. 6. The increased fouling tendency is as expected and must be overcome by more effective cleaning.
3.2 Macroscopic cleaning
The visualization of the macroscopic cleaning process is given in. In these images, the brightness corresponds to the level of remaining soil. It is evident that in the case of the optimized dimple the soil remains on the surface for the longest time. Nevertheless, all components are macroscopically clean after 150 s at the latest. The slow cleaning at the beginning of the experiment is related to the high initial surface soil coverage of the optimized dimple.
The development of the area-averaged surface soil coverage over time is depicted in Fig. 7. With the straight pipe and the basic dimple, cleaning was largely completed after the first 50 s; with the optimized dimple, it took about twice as long.
Based on the curves in Fig. 7, the soil removal rate \({\dot{m}}^{\prime \prime}_{s}\) can be determined. It corresponds to the gradient of the curves and is calculated via the finite difference quotient by
The resulting curves are displayed in Fig. 8. The removal rate is highest at the beginning of the experiments, decreases faster initially and levels off towards the end. A dedicated swelling phase, as observed in cleaning tests when removing a starch layer by Joppa et al. [32], does not occur in the present experiments with sour milk or at least it is too short to be noticed. The removal rate curve for the optimized dimple shows a peculiarity. After the initial decrease, a plateau is formed where the removal rate remains almost constant for about 50 s.
The cleaning process was further analyzed regarding cleaning time and mean cleaning rate. The cleaning time \({t}_{95}\) represents the time which is necessary to remove 95% of the initial mass of soil and is determined as
To further compare the cleaning experiments of the different surfaces, the mean cleaning rate \({R}_{95}\) was used. It was calculated via
using the ratio of the initial surface soil coverage and cleaning time.
The average cleaning time and mean cleaning rate are shown in Fig. 9.
The structuring of the surface leads to an increase in macroscopic cleaning time, where the optimized dimple structure leads to the longest cleaning time. This can be attributed to the fact, that it had the highest initial surface soil coverage. A one-way ANOVA was conducted to evaluate the effect of surface design on mean cleaning time. It revealed that there was a statistically significant effect on mean cleaning time [F(2, 9) = 15.94, p = 0.001] for the three different surface designs. Post-hoc comparisons using Tukey's test showed a significant difference in mean cleaning time at the p < 0.05 level between the straight reference pipe and the optimized dimple (p = 0.0008, 95% C.I. = [17.91, 52.97]). Although the optimized dimple required the longest cleaning time, the cleaning itself was slightly more efficient, indicated by the highest mean cleaning rate. It demonstrates that a larger amount of soil per minute was removed in the optimized dimple-structured pipe than in the straight pipe. However, a one-way ANOVA showed that these differences were not statistically significant, leading to the conclusion that the cleaning rates are comparable.
In order to compare the experimental results with the simulations performed by Joppa et al. [26], the mean cleaning rate of the macro-structured pipes is normalized with the mean cleaning rate of the straight pipe. This normalized mean cleaning rate \({r}_{95}\) can be compared to the overall cleaning performance \({E}_{\mathrm{oa}}\), which was used to assess the cleanability of film-like soils in the dimpled pipes by flow simulations. Both quantities are relative criteria allowing for evaluating the cleaning behavior of the macrostructures relative to the reference case of a straight pipe. Table 1 lists the respective values for the investigated surface structures.
Experimental and simulative results both show the same trend, i.e., the basic dimple structure leads to a reduction in cleaning performance, while the optimized dimple slightly improves it. A direct quantitative comparison of the two variables is not meaningful because they were determined differently. In the overall cleaning performance, all cleaning mechanisms were weighted equally, while the mean cleaning rate only reflects the cleaning behavior with respect to the specific cleaning mechanism in the experiments.
However, the comparison shows that the optimized dimple structure, which was developed exclusively by means of flow simulations, also has a positive effect on the cleaning performance in the macroscopic cleaning experiments.
3.3 Microbial cleaning
For applications in the food industry, the additively manufactured components must also ensure microbial cleanliness. This was evaluated after the cleaning experiments by probing with SHA agar as described before. The average residual microbial contamination of all surface structures is shown in Fig. 10.
In the straight pipe, on average, over 75% of the surface exhibited residual microbial contamination. This high value is related to the relatively high roughness of the wall surface, which makes it difficult or even impossible to remove microbial contamination. This emphasizes the importance of smooth surfaces and the need to reduce the roughness of 3D-printed parts to be used in the food industry. A one-way ANOVA showed a significant effect of the surface design on mean microbial contamination at the p < 0.05 level for the three designs tested [F(2, 15) = 15.97, p = 0.0002].
Tukey's range test for multiple comparisons found that the mean value of microbial contamination was significantly different between the basic dimple and the straight reference pipe (p = 0.007, 95% C.I. = [12.51%, 78.69%]) as well as between the optimized dimple and the straight reference pipe (p = 0.0001, 95% C.I. = [37.97%, 104.14%]).
These results indicate that the insertion of dimple structures into the pipe surface leads to a significant reduction in microbial contamination. While 32% of the agar’s surface was discolored in the pipes structured with the basic dimples, this value was reduced to only 6% for the optimized dimple. It should be noted that these pipes also exhibited the high roughness caused by the additive manufacturing process. Nevertheless, the results demonstrate the positive, relative effect of the macrostructures on microbial cleanability compared to a straight pipe of the same surface finish.
Remarkably, the optimized dimple structure of Joppa et al. [26] leads to the least residual microbial contamination. This contrasts with the macroscopic cleaning experiments, which predicted the longest cleaning time for the optimized dimple structure at comparable cleaning rates to the reference pipe. It may be attributed to the fact that a different removal mechanism acts on the protein soiling and bacterial contamination.
In the experiments, the protein deposit was removed very uniformly and continuously, i.e., viscous displacement and adhesive separation play only a minor role in the cleaning process. If this is considered, it seems appropriate to assume a cleaning by diffusive dissolution and cohesive separation of small particles, as reported by Xin et al. [35] for the removal of whey protein from stainless steel surfaces.
While the proteinaceous deposit is removed effectively and comparably fast from all surfaces, the bacterial spores remain on the pipe surfaces even after a longer cleaning cycle with higher mechanical action, i.e., fluid velocity, acting on them.
Lelièvre et al. [36] studied the influence of local wall shear stress and its fluctuation on the removal of bacteria from stainless steel surfaces. Their results indicate that the fluctuation of wall shear stress had a positive effect on microbial cleaning, regardless of the geometry, so that even regions with low mean wall shear stress could be cleaned successfully. Transferred to the microbial cleaning of structured pipes, this could indicate that the quantities relevant for cleaning fluctuate more intensively with the dimpled pipes than with the straight pipe. However, supporting simulation or experimental results proving this assumption are not available at the present time.
4 Conclusions
Within the present study, the cleaning of sour milk in dimple-structured pipes was investigated and the influence of the surface structure on the cleanability was assessed. The results show that the studied dimple structures increase the fouling tendency because the soil accumulates in the cavities. In consequence, macroscopic cleaning of the dimple-structured pipes required more time than for the straight pipe. However, this was mainly due to the higher initial surface soil coverage.
The main advantage of the dimple structures became apparent when evaluating microbial cleanliness. Both dimple shapes led to a significant reduction in microbial contamination, with the optimized dimple structure providing the best result.
From an industrial perspective, a tension exists between the higher fouling tendency, i.e., decreasing process performance and profit, and improved microbial cleanability, i.e., higher consumer safety, that should be considered when applying macro-structured surfaces.
The present cleaning experiments prove that the simulation results of Joppa et al. [26] are valid. Furthermore, they suggest that the proposed approach for evaluating the cleanability of film-like soils in pipes by flow simulation as well as the defined criterion for the cleaning performance are suitable.
Upcoming work will investigate the influence of a combination of surface finishing methods and macrostructures on the cleaning performance. This may further improve cleanability and make metal 3D printing competitive with established manufacturing processes. Moreover, the investigation of other promising surface structures, like protrusions, is planned.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Abbreviations
- D :
-
Diameter m
- E oa :
-
Overall cleaning performance %
- \(m^{\prime \prime}_{s}\) :
-
Surface soil coverage g/m2
- \({\dot{m}}^{\prime \prime}_s\) :
-
Soil removal rate g/(m2s)
- R 95 :
-
Mean cleaning rate g/(m2s)
- Ra :
-
Arithmetic profile roughness m
- r95 :
-
Normalized mean cleaning rate
- T :
-
Temperature \(^\circ \mathrm{C}\)
- t 95 :
-
Cleaning time when 5 percent of the initial soil remain s
- u :
-
Mean velocity m/s
- 0:
-
initial
References
Sacco E, Moon SK (2019) Additive manufacturing for space: status and promises. Int J Adv Manuf Technol 105:4123–4146. https://doi.org/10.1007/s00170-019-03786-z
Anton A, Reiter L, Wangler T et al (2021) A 3D concrete printing prefabrication platform for bespoke columns. Automation in Construction 122:103467. https://doi.org/10.1016/j.autcon.2020.103467
Kermavnar T, Shannon A, O’Sullivan KJ et al (2021) Three-Dimensional Printing of Medical Devices Used Directly to Treat Patients: A Systematic Review. 3D Print Addit Manuf 8:366–408. https://doi.org/10.1089/3dp.2020.0324
Bournias-Varotsis A, Han X, Harris RA, Engstrøm DS (2019) Ultrasonic additive manufacturing using feedstock with build-in circuitry for 3D metal embedded electronics. Addit Manuf 29:100799. https://doi.org/10.1016/j.addma.2019.100799
Gong G, Ye J, Chi Y et al (2021) Research status of laser additive manufacturing for metal: a review. J Market Res 15:855–884. https://doi.org/10.1016/j.jmrt.2021.08.050
Scheithauer U, Kordaß R, Noack K et al (2018) Potentials and Challenges of Additive Manufacturing Technologies for Heat Exchanger. IntechOpen
Sheikholeslami M, Gorji-Bandpy M, Ganji DD (2015) Review of heat transfer enhancement methods: Focus on passive methods using swirl flow devices. Renew Sustain Energy Rev 49:444–469. https://doi.org/10.1016/j.rser.2015.04.113
Qian B, Fan H, Liu G et al (2021) Microchannel Liquid-Cooled Heat Exchanger Based on a Nonuniform Lattice: Study on Structure Calculation, Formation Process, and Boiling Heat Transfer Performance. Materials 14:7248. https://doi.org/10.3390/ma14237248
Unger S, Beyer M, Gruber S et al (2019) Experimental study on the air-side thermal-flow performance of additively manufactured heat exchangers with novel fin designs. Int J Therm Sci 146:106074. https://doi.org/10.1016/j.ijthermalsci.2019.106074
Jafari D, Wits WW (2018) The utilization of selective laser melting technology on heat transfer devices for thermal energy conversion applications: A review. Renew Sustain Energy Rev 91:420–442. https://doi.org/10.1016/j.rser.2018.03.109
Geenen K, Röttger A, Theisen W (2017) Corrosion behavior of 316L austenitic steel processed by selective laser melting, hot-isostatic pressing, and casting. Mater Corros 68:764–775. https://doi.org/10.1002/maco.201609210
European Hygienic Engineering Design Group (2018) EHEDG Guidelines Doc. 8: Hygienic Design Principles, 3rd ed
Ferchow J, Hofmann U, Meboldt M (2020) Enabling Electropolishing of Complex Selective Laser Melting Structures. Procedia CIRP 91:472–477. https://doi.org/10.1016/j.procir.2020.02.201
Calvimontes A, Mauermann M, Bellmann C (2012) Topographical Anisotropy and Wetting of Ground Stainless Steel Surfaces. Materials 5:2773–2787. https://doi.org/10.3390/ma5122773
Ligrani P (2013) Heat Transfer Augmentation Technologies for Internal Cooling of Turbine Components of Gas Turbine Engines. Int J Rotating Mach 2013:275653. https://doi.org/10.1155/2013/275653
Kornev N, Hassel E, Herwig H et al (2005) Erhöhung des Wärmeüberganges durch Wirbelinduktion in Oberflächendellen. Forsch Ingenieurwes 69:90–100. https://doi.org/10.1007/s10010-004-0142-y
Turnow J, Kornev N, Zhdanov V, Hassel E (2012) Flow structures and heat transfer on dimples in a staggered arrangement. Int J Heat Fluid Flow 35:168–175. https://doi.org/10.1016/j.ijheatfluidflow.2012.01.002
Murcek R, Fuchs E, Boye A et al (2015) Effect of structured surfaces on fouling and cleaning behaviour in plate heat exchangers. In: Proceedings of the International Conference on Heat Exchanger Fouling and Cleaning - 2015. Enfield, Irland
Kasper R, Deponte H, Michel A et al (2018) Numerical investigation of the interaction between local flow structures and particulate fouling on structured heat transfer surfaces. Int J Heat Fluid Flow 71:68–79. https://doi.org/10.1016/j.ijheatfluidflow.2018.03.002
Kasper R, Turnow J, Kornev N (2019) Multiphase Eulerian–Lagrangian LES of particulate fouling on structured heat transfer surfaces. Int J Heat Fluid Flow 79:108462. https://doi.org/10.1016/j.ijheatfluidflow.2019.108462
Deponte H, Rohwer L, Augustin W, Scholl S (2019) Investigation of deposition and self-cleaning mechanism during particulate fouling on dimpled surfaces. Heat Mass Transf 55:3633–3644. https://doi.org/10.1007/s00231-019-02676-0
Deponte H, Kasper R, Schulte S et al (2020) Experimental and numerical approach to resolve particle deposition on dimpled heat transfer surfaces locally and temporally. Chem Eng Sci 227:115840. https://doi.org/10.1016/j.ces.2020.115840
Köhler H, Liebmann V, Joppa M et al (2021) On the Concept of Computational Fluid Dynamics-Based Prediction of Cleaning for Film-Like Soils. Heat Transf Eng 1–10. https://doi.org/10.1080/01457632.2021.1974180
Fryer PJ, Asteriadou K (2009) A prototype cleaning map: A classification of industrial cleaning processes. Trends Food Sci Technol 20:255–262. https://doi.org/10.1016/j.tifs.2009.03.005
Joppa M, Köhler H, Rüdiger F et al (2020) Prediction of Cleaning by Means of Computational Fluid Dynamics: Implication of the Pre-wetting of a Swellable Soil. Heat Transfer Eng 41:178–188. https://doi.org/10.1080/01457632.2018.1522096
Joppa M, Hanisch T, Mauermann M (2022) Methodology for the assessment of cleanability and geometry optimization using flow simulation on the example of dimple-structured pipe surfaces. Food Bioprod Process 132:141–154. https://doi.org/10.1016/j.fbp.2021.12.004
Pretorius C, Buys EM (2021) Extended shelf life milk processing: Effect of simulated cleaning in place on the germination and attachment of Bacillus cereus spores. Int J Dairy Technol 74:75–83. https://doi.org/10.1111/1471-0307.12744
Fan M, Phinney DM, Heldman DR (2015) Effectiveness of Rinse Water during In-Place Cleaning of Stainless Steel Pipe Lines. J Food Sci 80:E1490–E1497. https://doi.org/10.1111/1750-3841.12914
European Hygienic Engineering Design Group (2007) EHEDG Guidelines Doc. 2: A method for assessing the in-place cleanability of food processing equipment, 3rd ed
Mauermann M, Majschak J-P, Bley T et al (2012) Cleanability of food contact surfaces with water jets. Chem Ing Tec 84:1568–1574. https://doi.org/10.1002/cite.201100232
Helbig M, Föste H, Augustin W et al (2015) Description of the cleaning mechanism of a model food soil using an optical detection method and the FDG technique. In: Proceedings of the Heat Exchanger Fouling and Cleaning Conference XI. Enfield, Ireland
Joppa M, Köhler H, Rüdiger F et al (2017) Experiments and Simulations on the Cleaning of a Swellable Soil in Plane Channel Flow. Heat Transfer Eng 38:786–795. https://doi.org/10.1080/01457632.2016.1206420
Murcek R, Hölzel J, Köhler H et al (2021) Development of a quartz crystal sensor system to monitor local soil removal during cleaning in closed food processing lines. Food Bioprod Process 127:282–287. https://doi.org/10.1016/j.fbp.2021.03.011
Tukey JW (1949) Comparing Individual Means in the Analysis of Variance. Biometrics 5:99–114. https://doi.org/10.2307/3001913
Xin H, Chen XD, Özkan N (2004) Removal of a model protein foulant from metal surfaces. AIChE J 50:1961–1973. https://doi.org/10.1002/aic.10149
Lelièvre C, Legentilhomme P, Gaucher C et al (2002) Cleaning in place: effect of local wall shear stress variation on bacterial removal from stainless steel equipment. Chem Eng Sci 1287–1297. https://doi.org/10.1016/S0009-2509(02)00019-2
Funding
Open Access funding enabled and organized by Projekt DEAL. The IGF project (20790 BR) is supported by the Research Association of the Industrial Association for Food Technology and Packaging (IVLV e.V.) and is funded by the Federal Ministry for Economic Affairs and Climate Action via the AiF as part of the program for the promotion of industrial community research (IGF) based on a decision of the German Bundestag.
Author information
Authors and Affiliations
Contributions
Conceptualization, formal analysis and methodology: Tobias Hanisch, Matthias Joppa, Vincent Eisenrauch; Investigation and validation: Sebastian Jacob, Vincent Eisenrauch; Tobias Hanisch; Writing—original draft: Tobias Hanisch, Vincent Eisenrauch; Writing—review and editing: Matthias Joppa, Marc Mauermann; Resources and supervision: Marc Mauermann.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hanisch, T., Joppa, M., Eisenrauch, V. et al. Optimizing the macrostructure of 3D-printed pipe surfaces to improve cleanability. Heat Mass Transfer 60, 887–895 (2024). https://doi.org/10.1007/s00231-023-03387-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-023-03387-3