Abstract
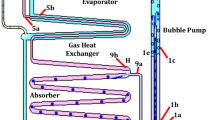
This research aims to develop diffusion absorption refrigeration system DARS' performance coefficients that can work especially with low-temperature heat sources. For this purpose, the properties of the used (Fe3O4-ammonia/water) nanoferrofluid as a refrigeration binary working solution were determined and examined its efficiency characteristics depending on separated thermophysical and visual inspection analyses. Later, in the absence and presence of an external magnetic field that affects the DARS' bubble pump-generator component, the validation of using the nanoferrofluid was experimentally investigated. Then an experimental study of obtained enhancement in DARS' performance was presented by using the solution with helium as an inert gas. No previous study has tested this nanoferrofluid based on the ammonia/water binary working solution under an external magnetic field effect as it is performed in this research. So this work can be considered an investigation of a new nanoferrofluid and a continuation of the previous studies. The experiments were performed on nanoferrofluids of 0.05 wt.% and 0.1 wt.% Fe3O4 nanoparticles concentrations in 300 ml NH3/H2O base-fluid with addition 1 wt.% concentration of polyvinyl pyrrolidone surfactant, which was equipped to DARS with and without an external magnetic field. Depending on the concentrations and presence or absence of the external magnetic field, the experiments are divided into five tests. According to the obtained results of the analyses, DARS with 0.1 wt.% nanoferrofluid under the external magnetic field was the best system with enhancements in COP, ECOP, reached 10.72%, 26.66%, respectively, and a decrease in the circulation ratio (f) by 2.70%.













Similar content being viewed by others
Abbreviations
- SHE:
-
Solution Heat Exchanger
- GHX:
-
Gas Heat Exchanger
- RHE:
-
Refrigerant Heat Exchanger
- PHE:
-
Plate Heat Exchanger
- DARS:
-
Diffusion Absorption Refrigeration System
- ARS:
-
Absorption Refrigeration System
- COP:
-
Coefficient of Performance
- ECOP:
-
Exergetic Coefficient of Performance
- f :
-
Circulation Ratio
- CNT:
-
Carbon Nanotube
- \(\dot{Q}\) :
-
Heat transfer rate (kJ s−1)
- T :
-
Temperature (oC)
- \(\dot{m}\) :
-
Mass flow rate (kg s−1)
- E :
-
Specific exergy (kJ kg−1)
- h :
-
Specific enthalpy (kJ kg−1)
- s :
-
Specific entropy (kJ kg−1 K−1)
- ig:
-
Inert gas
- ws:
-
Weak working solution
- rs:
-
Rich working solution
- gen:
-
Generator
- cond:
-
Condenser
- evap:
-
Evaporator
- rect:
-
Rectifier
- pip,r:
-
Pipelines and reservoir
- dest:
-
Destruction
- co:
-
Coolant
- sol:
-
Working solution
- H2O:
-
Water
- NH3 :
-
Ammonia
References
Von Platen BC, Munters CG (1928) Refrigerator, US Patent 1, 685–764
Narayankhekar KG, Maiya MP (1985) Investigation on triple fluid vapor absorption refrigerator. Int J Refrig 8:335–342
Gutiérrez F (1998) Behavior of a household absorption diffusion refrigerator adapted to autonomous solar operation. Solar Energ 40:17
Smirnov GF, Bukraba MA, Fattuh T, Nabulsi B (1996) Domestic refrigerators with absorption-diffusion units and heat transfer panels. Int J Refr 19:208–18
Shelton SV, White S (2002) Bubble pump design for single pressure absorption refrigeration cycles. ASHRAE Trans 108:1–10
Benhmidene A, Chaouachi B, Gabsi S, Bourouis M (2011) Modelling of heat flux received by a bubble pump of absorption-diffusion refrigeration cycle. Heat Mass Transf 47:1341–1347
Benhmidene A, Chaouachi B, Bourouis M, Gabsi S (2011) Effect of operating conditions on the performance of the bubble pump of absorption-diffusion refrigeration cycles. Thermal Sci 15(3):793–806
Dammak N, Chaouachi B, Gabsi S, Bourouis M (2010) Optimization of the geometrical parameters of a solar bubble pump for absorption-diffusion cooling systems. Am J Eng Appl Sci 3:693–698
Abu-Mulaweh HI, Mueller DW, Wegmann B, Speith K, Boehne B (2011) Design of a bubble pump cooling system demonstration unit. Int J Thermal and Environmental Eng 2:1–8
Abduwadood SS, Akeel MAM (2012) Experimental investigation of water vapour-bubble pump characteristics and its mathematical reconstruction. Engineering and Technical Journal 30:1870–1885
McLinden MO, Lemmon EW, Jacobsen RT (1998) Thermodynamic properties for alternative refrigerants. Int J Refrig 21:332–338
Ziegler B, Trepp C (1984) Equation of state for ammonia-water mixtures. Int J Refrig 7:101–106
El-Sayed YM, Tribus M (1985) Thermodynamic properties of water-ammonia mixtures: theoretical implementation for use in power cycle analysis. ASME Publications 1:89–95
Herold KE, Han K, Moran AMMWATMJ (1988) A computer program for calculating the thermodynamic properties of ammonia and water mixtures using a Gibbs free energy formulation. ASME Publications 1988(4):65–75
Bourseau P, Bugarel R (1986) Réfrigération par cycle à absorption–diffusion: comparaison des performances des systèmes NH3–H2O et NH3–NaSCN. Int J Refrig 9:206–214
Acuña A, Velazquez N, Cerezo J (2013) Energy analysis of a diffusion absorption cooling system using lithium nitrate, sodium thiocyanate and water as absorbent substance and ammonia as the refrigerant. Appl Therm Eng 51:1273–1281
Lee JK, Koo J, Hong H, Kang YT (2010) The effects of nanoparticles on absorption heat and mass transfer performance in NH3/H2O binary nanofluids. Int J Refrig 33(2010):269–275
Yang L, Du K, Niu XF, Cheng B, Jiang YF (2011) Experimental study on enhancement of ammonia–water falling film absorption by adding nanoparticles. Int J Refrig 34(2011):640–647
Wu WD, Pang CW, Sheng W (2010) Enhancement on NH3/H2O bubble absorption in binary nanofluids by mono nano Ag. J Chem Ind Eng 61(2010):1112–1117
Cuenca Y, Vernet A, Valle`s M (2014) Thermal conductivity enhancement of the binary mixture (NH3+LiNO3) by the addition of CNTs. Int J Refrig 41:113–120
Mohammed HI, Giddings D, Walker GS (2019) Experimental investigation of nanoparticles concentration, boiler temperature and flow rate on flow boiling of zinc bromide and acetone solution in a rectangular duct. Int J Heat Mass Transf 130(2019):710–721
Mohammed HI, Giddings D, Walker GS (2020) Thermo-physical properties of the nano-binary fluid (acetone–zinc bromide-ZnO) as a low temperature operating fluid for use in an absorption refrigeration machine. Heat and Mass Transf 56:1037–1044
Wang S, Liu Y, Chen Y, Wang Q, Xu X, Chen G, Deng S (2019) Experimental investigations on the temperature lift performance of a novel diffusion absorption heat transformer. Energy 170:906–914
Kim JK, Lee JK, Kang YT (2007) Absorption performance enhancement by nanoparticles and chemical surfactants in binary nanofluids. Int J Refrigeration 30:50–57
Hodenius MA, Niendorf T, Krombach GA, Richtering W, Eckert T, Lueken H, Speldrich M, Günther RW, Baumann M, Soenen SJ, De Cuyper M, SchmitzRode T (2008) Synthesis, physicochemical characterization and MR relaxometry of aqueous ferrofluids. J Nanosci Nanotechnol 8(2008):2399–2409
Nourafkana E, Asachia M, Gaoa H, Razaa G, Wena D (2017) Synthesis of stable iron oxide nanoparticle dispersions in high ionic media. J Ind Eng Chem 50(2017):57–71
Ranjan G, Swarnendu S, Puri IK (2004) Heat transfer augmentation using a magnetic fluid under the influence of a line dipole. J Magn Magn Mater 271(2004):63–73
Li Q, Xuan YM (2009) Experimental investigation on heat transfer characteristics of magnetic fluid flow around a fine wire under the influence of an external magnetic field. Exp Thermal Fluid Sci 33(2009):591–596
Yu W, Xie HQ, Chen LF, Li Y (2010) Enhancement of thermal conductivity of kerosene-based Fe3O4 nanofluids prepared via phase-transfer method. Colloids Surf A 335(2010):109–113
Pinto RV, Fiorelli FAS (2016) Review of the mechanisms responsible for heat transfer enhancement using nanofluids. Appl Therm Eng 108:720–739
Olle B, Bucak S, Tracy CH, Bromberg L, Hatton AT (2006) Enhancement of oxygen mass transfer using functionalized magnetic nanoparticles. Ind Eng Chem Res 45(2006):4355–4363
Srinivas K, Rajagopal M, Suresh AK (2007) Synthesis, characterization and testing for ferrofluids for mass transfer intensification. Int J Refrig 5(2007):1913–1928
Takahashi M, Shinbo K, Ohkawa R, Matsuzaki M, Inoue A (1993) Nucleate pool boiling heat transfer of magnetic fluid in a magnetic field. J Magn Magn Mater 122(1993):301–304
Bashtovoi VG, Challant G, Yu VO (1993) Boiling heat transfer in magnetic fluids. J Magn Magn Mater 122(1993):305–308
Kamiyama S, Ishimoto J (1995) Boiling two-phase flows of magnetic fluid in a nonuniform magnetic field. J Magn Magn Mater 149(1995):125–131
Liu J, Gu J, Lian Z, Liu H (2004) Experiments and mechanism analysis of pool boiling enhancement with water-based magnetic fluid. Heat Mass Transfer 41(2004):170–175
Niu XF, Du K, Du SX (2007) Numerical analysis of falling film absorption with ammonia–water in magnetic field. Appl Therm Eng 27:2059–2065
Wu WD, Liu G, Chen SX, Zhang H (2013) Nanoferrofluid addition enhances ammonia/water bubble absorption in an external magnetic field. Energy and Buildings 57:268–277
Kouremenos DA, Stegou-Sagia A (1988) Use of helium instead of hydrogen in inert gas absorption refrigeration. Int J Refrig 11:336–341
Kouremenos DA, Stegou-Sagia A, Antonopoulos KA (1994) Three-dimensional evaporation process in aqua-ammonia absorption refrigerators using helium as inert gas. Int J Refrig 17:58–67
Herold KE, Chen J (1994) Diffusion–absorption heat pump. Annual Report for Gas Research Institute, USA. Available from: http://adsabs.harvard.edu/abs/1994umd.. rept…..H.
Zohar A, Jelinek M, Levy A, Borde I (2005) Numerical investigation of a diffusion absorption refrigeration cycle. Int J Refrig 28:515–525
Sözen A, Menlik T, Özbaş E (2012) The effect of ejector on the performance of diffusion absorption refrigeration systems: An experimental study. Appl Therm Eng 33–34:44–53. https://doi.org/10.1016/j.applthermaleng.2011
Sözen A, Özbaş E, Menlik T, Çakır MT, Gürü M, Boran K (2014) Improving the thermal performance of diffusion absorption refrigeration system with alumina nanofluids: An experimental study. Int J Refrig 44:73–80. https://doi.org/10.1016/j.ijrefrig.2014.04.01
Gürbüz EY, Sözen A, Keçebaş A, Özbaş E (2020) Experimental and numerical investigation of diffusion absorption refrigeration system working with ZnOAl2O3 and TiO2 nanoparticles added ammonia/water nanofluids. Experimental Heat Transfer. https://doi.org/10.1080/08916152.2020.1838668
Gürbüz EY, Sözen A, Özbaş E (2019) Upgrading of Performance of Diffusion Absorption Refrigeration System by Using TiO2 Nanoparticles. Conference: 2nd Internati̇onal conference on energy research, April 2019
Özbas E (2018a) Experimental Study of Thermal Performance and Pressure Differences of Different Working Fluids in Two-phase Closed Thermosyphons Using Solar Energy March 2018. J Polytechnic. https://doi.org/10.2339/politeknik.407258
Özbas E. (2018b) Experimental Study Of Diffusion Absorption Refrigeration Systems Using Solar Energy. January 2018. J Polytechnic. https://doi.org/10.2339/politeknik.385931
Zohar A, Jelinek M, Levy A, Borde I (2007) The influence of diffusion absorption refrigeration cycle configuration on the performance. Appl Therm Eng 27:2213–2219
Fluke Corporation (2021) Fluke 43B/003 Power Quality Analyzer, web-based product information accessed at https://www.fluke-direct.com/product/fluke-43b-003-power-quality-analyzer. Last Accessed January (2021)
Mehyo M, Ozcan H (2021) Thermophysical Properties of Nanoferrofluid (Fe3O4 –Acetone/Znbr2) as a Working Fluid for Use in Absorption Refrigeration Applications, November 2021. Int J Thermodyn. https://doi.org/10.5541/ijot.949012
Zhou SQ, Ni R (2008) Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl Phys Lett 92(9):093123
Sekhar YR, Sharma KV (2015) Study of viscosity and specific heat capacity characteristics of water-based Al2O3nanofluids at low particle concentrations. J ExpNanosci 10(2):86–102
Ali A, Zafar H, Zia M, Haq IU, Phull AR, Ali JS, Hussain A (2016) Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 2016(9):49–67
Sözen A, Özbaş E, Menlik T, İskender Ü, Kılınç C, Çakır MT (2015) Performance investigation of a diffusion absorption refrigeration system using nano-size alumina particles in the refrigerant. Int J Exergy 18(4):443–461
Pitatowsky I, Rivera W, Romero RJ (2001) Thermodynamic analysis of monomethylamine-water solutions in a single-stage solar absorption refrigeration cycle at low generator temperatures. Sol Energy Mater Sol Cells 70:287–300
Rodríguez-Muñoz JL, Belman-Flores JM (2014) Review of diffusion–absorption refrigeration technologies. Renew Sustain Energy Rev 30:145–153
Yıldız A, Ersöz MA (2013) Energy and exergy analyses of the diffusion absorption refrigeration system. Energy xxx 2013:1–9
Mehyo M, Ozcan H, Hassan AHA (2018) Thermodynamic Analysis of a Power Plant Waste Heat Driven Absorption Refrigeration System. Proc 4th World Congress on Mechanical, Chem Mater Eng (MCM'18) Madrid, Spain – August 16 – 18, 2018
Zemaitis JF, Clark DM, Rafal M, Scrivner NC (1986) Handbook of aqueous electrolyte thermodynamics. American Institute of Chemical Engineers, New York
Zemaitis JF, Clark DM, Rafal M, Scrivner NC (1986) Handbook of aqueous electrolyte thermodynamics. American Institute of Chemical Engineers, New York
Magnet Market, web-based product information accessed at https://www.magnetmarket.com.tr/boy-40mm-x-en-10mm-x-kalinlik-5mm-neodymium-magnet-html. Last Accessed January (2021)
Acknowledgements
This study was supported by The Management Unit of Scientific Research Projects of Ondokuz Mayis University under Project PYO.MUH.1904.19.011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehyo, M., Özbaş, E. & Özcan, H. Performance investigation of utilizing nanoferrofluid as a working solution in a diffusion absorption refrigeration system under an external magnetic field effect. Heat Mass Transfer 58, 2107–2128 (2022). https://doi.org/10.1007/s00231-022-03214-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-022-03214-1