Abstract
Background
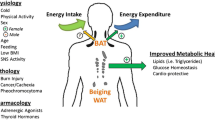
Brown adipose tissue (BAT) has emerged as a potential therapeutic target for metabolic disorders due to its thermogenic and anti-obesity properties. β3-adrenergic receptor (β3-AR) agonists have also gained attention as potential agents for BAT activation and metabolic regulation. Mirabegron, a selective β3-AR-agonist used clinically for overactive bladder syndrome, has been explored for its utility in metabolic disorders. However, the controversy surrounding the ability of mirabegron to activate BAT to accelerate metabolism requires further investigation. The aim of this systematic review is to characterize comprehensively the impact of mirabegron on human BAT and its metabolism.
Methods
We searched PubMed Central, Web of Science, Embase, and Cochrane Library databases for relevant papers published from the date of database inception to March 2023 for systematic reviews and meta-analyses. We extracted data on primary outcome indicators such as BAT volume, BAT activity, body temperature, and resting energy expenditure (REE), as well as secondary outcome indicators such as heart rate (HR), diastolic blood pressure (DBP), systolic blood pressure (SBP), non-esterified fatty acids (NEFA), blood glucose, and blood insulin from relevant studies. For studies that did not provide suitable data for meta-analysis, we used narrative data synthesis. For studies that provided suitable data for meta-analysis, we conducted meta-analysis using RevMan 5.4 software.
Results
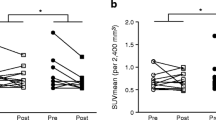
We reviewed 10 papers and included 6 in our meta-analysis. Our findings revealed no significant changes in BAT volume (p = 0.72) or blood glucose (p = 0.52) with mirabegron when compared to the placebo or pre-dose population. However, patients showed significant increases in BAT activity (p < 0.01), blood NEFA (p < 0.01), body temperature (p < 0.01), REE (p < 0.01), HR (p < 0.01), DBP (p < 0.01), SBP (p = 0.25), and blood insulin (p < 0.01).
Conclusion
Through our meta-analysis of 6 papers, we found that mirabegron has the potential to increase human BAT activity, REE, NEFA content, body temperature, HR, blood pressure, and blood insulin content. These effects may lead to reductions in blood glucose levels in obese/overweight and diabetic patients. Additionally, the activation of BAT by mirabegron could represent a novel approach for treating obesity, diabetes, and cardiovascular disease.
Trial registration number and date
CRD42023413446, 04/11/2023.










Similar content being viewed by others
Availability of data and materials
All of the material is owned by the authors and/or no permissions are required.
References
Medanić D, Pucarin-Cvetković J (2012) Obesity–a public health problem and challenge. Acta Med Croatica 66(5):347–355
Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15(5):288–298. https://doi.org/10.1038/s41574-019-0176-8
Trayhurn P (2022) Brown adipose tissue: a short historical perspective. In: Guertin DA, Wolfrum C (eds) Brown Adipose Tissue: Methods and Protocols. Springer, US, New York, NY, pp 1–18
Rasmussen AT (1922) The glandular status of brown multilocular adipose tissue. Endocrinology 6(6):760–770. https://doi.org/10.1210/endo-6-6-760
Aherne W, Hull D (1966) Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol 91(1):223–234. https://doi.org/10.1002/path.1700910126
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360(15):1518–1525. https://doi.org/10.1056/NEJMoa0808949
O’Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA et al (2020) Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Investig 130(5):2209–2219. https://doi.org/10.1172/jci131126
Li J, Zuo R, Schoepf UJ, Griffith JP 3rd, Wu S, Zhou C et al (2023) Development and validation of a nonenhanced CT based radiomics model to detect brown adipose tissue. Theranostics 13(5):1584–1593. https://doi.org/10.7150/thno.81789
Chen Z, Kang Y (2023) Cold snap for cancer: cold-induced brown fat thermogenesis starves tumor growth. Signal Transduct Target Ther 8(1):10. https://doi.org/10.1038/s41392-022-01284-5
Inoue T, Fu B, Nishio M, Tanaka M, Kato H, Tanaka M, et al (2023) Novel therapeutic potentials of taxifolin for obesity-induced hepatic steatosis, fibrogenesis, and tumorigenesis. Nutrients 15(2). https://doi.org/10.3390/nu15020350
Heaton GM, Wagenvoord RJ, Kemp A Jr, Nicholls DG (1978) Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem 82(2):515–521. https://doi.org/10.1111/j.1432-1033.1978.tb12045.x
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360(15):1500–1508. https://doi.org/10.1056/NEJMoa0808718
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359. https://doi.org/10.1152/physrev.00015.2003
Liu X, Pérusse F, Bukowiecki LJ (1998) Mechanisms of the antidiabetic effects of the beta 3-adrenergic agonist CL-316243 in obese Zucker-ZDF rats. Am J Physiol 274(5):R1212–R1219. https://doi.org/10.1152/ajpregu.1998.274.5.R1212
Goulooze SC, Cohen AF, Rissmann R (2015) Mirabegron. Br J Clin Pharmacol 80(4):762–764. https://doi.org/10.1111/bcp.12647
Cero C, Lea HJ, Zhu KY, Shamsi F, Tseng YH, Cypess AM (2021) β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 6(11). https://doi.org/10.1172/jci.insight.139160
Weyer C, Tataranni PA, Snitker S, Danforth E Jr, Ravussin E (1998) Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective beta3-adrenoceptor agonist in humans. Diabetes 47(10):1555–1561. https://doi.org/10.2337/diabetes.47.10.1555
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Clinical research ed) 372. https://doi.org/10.1136/bmj.n160
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds (2023) Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Available from www.training.cochrane.org/handbook. Accessed 2023
Wells GA, Wells G, Shea B, Shea B, O’Connell D, Peterson J et al (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses
Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J et al (2011) GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol 64(12):1277–82. https://doi.org/10.1016/j.jclinepi.2011.01.011
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383–94. https://doi.org/10.1016/j.jclinepi.2010.04.026
Fischer JGW, Maushart CI, Becker AS, Müller J, Madoerin P, Chirindel A et al (2020) Comparison of [18F]FDG PET/CT with magnetic resonance imaging for the assessment of human brown adipose tissue activity. EJNMMI Res 10(1). https://doi.org/10.1186/s13550-020-00665-7
Loeliger RC, Maushart CI, Gashi G, Senn JR, Felder M, Becker AS et al (2021) Relation of diet-induced thermogenesis to brown adipose tissue activity in healthy men. Am J Physiol Endocrinol Metab 320(1):E93–E101. https://doi.org/10.1152/AJPENDO.00237.2020
O’Mara A, Cypess A, Cero C, Johnson JW, Linderman JD, Leitner B et al (2018) Physiological responses to daily use of beta-three adrenergic receptor agonist, mirabegron. Diabetes 67:A307. https://doi.org/10.2337/db18-1146-P
Ying Z, Van Eenige R, Janssen L, Berbée JFP, Boon M, Kooijman S et al (2020) Brown adipose tissue activation with mirabegron enhances fat oxidation in APOE*3-leiden.CETP mice and humans. Atherosclerosis 315:e99. https://doi.org/10.1016/j.atherosclerosis.2020.10.304
Baskin AS, Linderman JD, Brychta RJ, McGehee S, Anflick-Chames E, Cero C et al (2018) Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by a beta 3-adrenergic receptor agonist. Diabetes 67(10):2113–2125. https://doi.org/10.2337/db18-0462
Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA et al (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21(1):33–38. https://doi.org/10.1016/j.cmet.2014.12.009
Finlin BS, Memetimin H, Zhu B, Confides AL, Vekaria HJ, El Khouli RH et al (2020) The beta 3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Investig 130(5):2319–2331. https://doi.org/10.1172/JCI134892
Loh RKC, Formosa MF, La Gerche A, Reutens AT, Kingwell BA, Carey AL (2019) Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes Obes Metab 21(2):276–284. https://doi.org/10.1111/dom.13516
Nahon KJ, Janssen LGM, Sardjoe Mishre ASD, Bilsen MP, van der Eijk JA, Botani K et al (2020) The effect of mirabegron on energy expenditure and brown adipose tissue in healthy lean South Asian and Europid men. Diabetes Obes Metab 22(11):2032–2044. https://doi.org/10.1111/dom.14120
Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA et al (2016) Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab 24(2):210–22. https://doi.org/10.1016/j.cmet.2016.07.014
Holstila M, Pesola M, Saari T, Koskensalo K, Raiko J, Borra RJ et al (2017) MR signal-fat-fraction analysis and T2* weighted imaging measure BAT reliably on humans without cold exposure. Metab Clin Exp 70:23–30. https://doi.org/10.1016/j.metabol.2017.02.001
Labbé SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R et al (2015) In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J 29(5):2046–2058. https://doi.org/10.1096/fj.14-266247
Acosta FM, Martinez-Tellez B, Blondin DP, Haman F, Rensen PCN, Llamas-Elvira JM et al (2019) Relationship between the daily rhythm of distal skin temperature and brown adipose tissue (18)F-FDG uptake in young sedentary adults. J Biol Rhythms 34(5):533–550. https://doi.org/10.1177/0748730419865400
Haq T, Crane JD, Kanji S, Gunn E, Tarnopolsky MA, Gerstein HC et al (2017) Optimizing the methodology for measuring supraclavicular skin temperature using infrared thermography; implications for measuring brown adipose tissue activity in humans. Sci Rep 7(1):11934. https://doi.org/10.1038/s41598-017-11537-x
Law JM, Morris DE, Robinson L, Randell T, Denvir L, Symonds ME et al (2021) Reduced brown adipose tissue-associated skin temperature following cold stimulation in children and adolescents with type 1 diabetes. Pediatr Diabetes 22(3):407–416. https://doi.org/10.1111/pedi.13163
Martinez-Tellez B, Perez-Bey A, Sanchez-Delgado G, Acosta FM, Corral-Perez J, Amaro-Gahete FJ et al (2019) Concurrent validity of supraclavicular skin temperature measured with iButtons and infrared thermography as a surrogate marker of brown adipose tissue. J Therm Biol 82:186–196. https://doi.org/10.1016/j.jtherbio.2019.04.009
Sarasniemi JT, Koskensalo K, Raiko J, Nuutila P, Saunavaara J, Parkkola R et al (2018) Skin temperature may not yield human brown adipose tissue activity in diverse populations. Acta Physiol (Oxf) 224(3). https://doi.org/10.1111/apha.13095
Symonds ME, Henderson K, Elvidge L, Bosman C, Sharkey D, Perkins AC et al (2012) Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. J Pediatr 161(5):892–898. https://doi.org/10.1016/j.jpeds.2012.04.056
Björntorp P, Bergman H, Varnauskas E (1969) Plasma free fatty acid turnover rate in obesity. Acta Med Scand 185(4):351–356. https://doi.org/10.1111/j.0954-6820.1969.tb07347.x
Boden G (1997) Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46(1):3–10
Guo SX, Yan YZ, Mu LT, Niu Q, He J, Liu JM et al (2015) Association of serum free fatty acids with hypertension and insulin resistance among rural Uyghur adults in far western China. Int J Environ Res Public Health 12(6):6582–6590. https://doi.org/10.3390/ijerph120606582
Stojiljkovic MP, Zhang D, Lopes HF, Lee CG, Goodfriend TL, Egan BM (2001) Hemodynamic effects of lipids in humans. Am J Physiol Regul Integr Comp Physiol 280(6):R1674–R1679. https://doi.org/10.1152/ajpregu.2001.280.6.R1674
Zhao XY, Liu Y, Zhang X, Zhao BC, Burley G, Yang ZC et al (2023) The combined effect of metformin and mirabegron on diet-induced obesity. MedComm 4(2). https://doi.org/10.1002/mco2.207
Hao L, Scott S, Abbasi M, Zu Y, Khan MSH, Yang Y et al (2019) Beneficial Metabolic Effects of mirabegron in vitro and in high-fat diet-induced obese mice. J Pharmacol Exp Ther 369(3):419–427. https://doi.org/10.1124/jpet.118.255778
Roberts-Toler C, O’Neill BT, Cypess AM (2015) Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity (Silver Spring, Md) 23(9):1765–1770. https://doi.org/10.1002/oby.21134
Ito H, Matsuo T, Mitsunari K, Ohba K, Miyata Y (2022) Impact of mirabegron administration on the blood pressure and pulse rate in patients with overactive bladder. Medicina (Kaunas, Lithuania) 58(6). https://doi.org/10.3390/medicina58060825
Bundgaard H, Axelsson Raja A, Iversen K, Valeur N, Tønder N, Schou M et al (2022) Hemodynamic effects of cyclic guanosine monophosphate-dependent signaling through β3 adrenoceptor stimulation in patients with advanced heart failure: a randomized invasive clinical trial. Circ Heart Fail 15(7). https://doi.org/10.1161/circheartfailure.121.009120
Funding
The study was not funded.
Author information
Authors and Affiliations
Contributions
Lili Ma's main contributions are literature searching, literature screening, data extraction, and paper writing. Lianqiu Xiong's main contributions are data extraction. Guang Hang's main contribution is the verification of data and the revision of papers. Lili Ma prepared Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 and Tables 1, 2, and 3 was produced by Lianqiu Xiong and Gang Huang. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Search Algorithm in PubMed:
(((((((((((((((((“mirabegron” [Supplementary Concept]) OR (Betmiga[Title/Abstract])) OR (2-(2-aminothiazol-4-yl)-4′-(2-((2-hydroxy-2-phenylethyl)amino)ethyl)acetanilide[Title/Abstract])) OR (Betanis[Title/Abstract])) OR (YM 178[Title/Abstract])) OR (YM-178[Title/Abstract])) OR (2 (2 amino 1, 3 thiazol 4 yl) n [4 [2 [ (2 hydroxy 2 phenylethyl) amino] ethyl] phenyl] acetamide)) OR (2 (2 aminothiazol 4 yl) 4′ [2 [ (2 hydroxy 2 phenylethyl) amino] ethyl] acetanilide)) OR (2 amino n [4 [2 [ [2 hydroxy 2 phenylethyl] amino] ethyl] phenyl] 4 thiazoleacetamide)) OR (Betanis[Title/Abstract])) OR (betmiga[Title/Abstract])) OR (myrbetriq[Title/Abstract])) OR (n [4 [2 (2 hydroxy 2 phenylethylamino) ethyl] phenyl] 2 (2 aminothiazol 4 yl) acetamide)) OR (sc 211912[Title/Abstract])) OR (sc211912[Title/Abstract])) OR (ym 178[Title/Abstract])) OR (ym178[Title/Abstract])) AND (((((((((Adipose Tissue, Brown[MeSH Terms]) OR (Tissue, Brown Adipose[Title/Abstract])) OR (BAT[Title/Abstract])) OR (BAT[Title/Abstract])) OR (Fat, Brown[Title/Abstract])) OR (Hibernating Gland[Title/Abstract])) OR (BAT tissue[Title/Abstract])) OR (BATty tissue[Title/Abstract])) OR (fatty tissue, brown[Title/Abstract]))
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, L., Xiong, L. & Huang, G. Effects of mirabegron on brown adipose tissue and metabolism in humans: A systematic review and meta-analysis. Eur J Clin Pharmacol 80, 317–333 (2024). https://doi.org/10.1007/s00228-023-03614-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03614-0