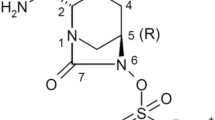
Abstract
Purpose
A series of iterative population pharmacokinetic (PK) modeling and probability of target attainment (PTA) analyses based on emerging data supported dose selection for aztreonam-avibactam, an investigational combination antibiotic for serious Gram-negative bacterial infections.
Methods
Two iterations of PK models built from avibactam data in infected patients and aztreonam data in healthy subjects with “patient-like” assumptions were used in joint PTA analyses (primary target: aztreonam 60% fT > 8 mg/L, avibactam 50% fT > 2.5 mg/L) exploring patient variability, infusion durations, and adjustments for moderate (estimated creatinine clearance [CrCL] > 30 to ≤ 50 mL/min) and severe renal impairment (> 15 to ≤ 30 mL/min). Achievement of > 90% joint PTA and the impact of differential renal clearance were considerations in dose selection.
Results
Iteration 1 simulations for Phase I/IIa dose selection/modification demonstrated that 3-h and continuous infusions provide comparable PTA; avibactam dose drives joint PTA within clinically relevant exposure targets; and loading doses support more rapid joint target attainment. An aztreonam/avibactam 500/137 mg 30-min loading dose and 1500/410 mg 3-h maintenance infusions q6h were selected for further evaluation. Iteration 2 simulations using expanded PK models supported an alteration to the regimen (500/167 mg loading; 1500/500 mg q6h maintenance 3-h infusions for CrCL > 50 mL/min) and selection of doses for renal impairment for Phase IIa/III clinical studies.
Conclusion
A loading dose plus 3-h maintenance infusions of aztreonam-avibactam in a 3:1 fixed ratio q6h optimizes joint PTA. These analyses supported dose selection for the aztreonam-avibactam Phase III clinical program. Clinical trial registration: NCT01689207; NCT02655419; NCT03329092; NCT03580044.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aztreonam-avibactam is an investigational fixed-dose combination antibiotic for serious infections caused by Gram-negative bacteria for which there are limited or no treatment options. Carbapenem-resistant Enterobacterales (CRE), including those that produce Ambler class B metallo-β-lactamases (MBLs), are an emerging threat for which treatment options are severely limited [1,2,3,4]. Aztreonam is an established monobactam (β-lactam), which retains activity in the presence of Ambler class B MBLs but is vulnerable to other β-lactamases which can be inhibited by avibactam, a first-in-class β-lactamase inhibitor. Avibactam has also been successfully partnered with ceftazidime (in a 4:1 fixed-dose ratio) for the treatment of infections caused by non-MBL CRE and other Gram-negative bacteria [5,6,7,8].
Initial clinical evaluations of the aztreonam-avibactam combination consisted of a Phase I dose-finding, safety, and pharmacokinetic (PK) trial in healthy subjects (NCT01689207) and a Phase IIa trial (REJUVENATE [NCT02655419]) in patients with complicated intra-abdominal infection (cIAI) [9, 10]. Two further confirmatory PK trials (NCT04486625 and NCT04973826) and two Phase III trials in patients with cIAI, complicated urinary tract infections (cUTI), hospital-acquired pneumonia and ventilator-associated pneumonia, and bloodstream infections caused by Gram-negative pathogens, including MBL-producing pathogens (REVISIT [NCT03329092] and ASSEMBLE [NCT03580044]), have recently been completed [11].
Clinical development of aztreonam-avibactam has been streamlined by leveraging preclinical and in vitro microbiological data [12,13,14,15], prior pharmacokinetic (PK) knowledge of aztreonam monotherapy [16,17,18,19], and avibactam population PK models built during the ceftazidime-avibactam clinical development program [5, 20,21,22,23,24]. Dose regimen selection for the aztreonam-avibactam clinical program was guided by approved dosing recommendations for aztreonam monotherapy, in vitro minimum inhibitory concentration (MIC) distributions for aztreonam and avibactam in combination against target pathogens, and simulations of joint probability of PK-pharmacodynamic (PD) target attainment (PTA) evaluating a range of dosing scenarios, balanced with exposures selected to limit potential adverse effects.
For β-lactam/β-lactamase inhibitor combinations, simultaneous achievement of both drugs’ respective plasma targets (referred to as joint target attainment) is required for antibacterial efficacy. For time-dependent drugs such as β-lactams, the PK/PD driver for efficacy is the proportion of the dosing interval that free plasma concentrations exceed the MIC (i.e. %fT > MIC). For avibactam, the time that free plasma concentrations exceed a critical threshold concentration (%fT > CT) is the PK/PD driver associated with efficacy in combinations with aztreonam or the cephalosporins ceftazidime and ceftaroline [13]. However, the threshold concentration required to protect the partner β-lactam varies depending on the combination [25]. Notably, the target for avibactam with aztreonam (50% fT > 2.5 mg/L) is 2.5-fold higher than for avibactam with ceftazidime (50% fT > 1 mg/L) [12, 13, 25]; this has fundamental implications for dosing of the respective combinations. Aztreonam is eliminated by both renal and extra-renal routes (approximately 70% and 30%, respectively) [26, 27], and dose adjustments are made for patients with severe renal impairment, defined as estimated creatinine clearance (CrCL) < 30 mL/min [28, 29]. In contrast, avibactam is almost entirely eliminated by renal clearance (97%), and doses of ceftazidime-avibactam are therefore modified for patients with estimated CrCL < 50 mL/min [30, 31]. Since the extent of renal clearance of avibactam is greater than that of aztreonam, understanding the relationships between renal function and exposures of both drugs has been an important aspect of the development program.
This paper describes the process of iterative population PK modeling and PTA analyses which, as additional PK and clinical data became available, supported dose selection and modification/optimization for the aztreonam-avibactam Phase II and Phase III clinical program. This included evaluation of loading doses and extended infusions, and dose adjustments for patients with moderate and severe renal impairment, accounting for the differential renal clearance of aztreonam and avibactam.
Methods
Overview and chronology of population PK modeling analyses
The aztreonam-avibactam clinical trial program has evaluated a range of intravenous dosing regimens (Table 1); an iterative series of population PK modeling and simulation analyses based on emerging data (Table 2) supported selection of the doses for the final cohorts of a Phase I “first-in-human” trial and for the Phase IIa and Phase III trials of aztreonam-avibactam. The Phase I trial was a three-part, placebo-controlled safety and PK evaluation of the combination of aztreonam with avibactam in healthy subjects [9]. In Part A, single doses of aztreonam 2000 mg with and without avibactam 600 mg were administered by 1-h infusions. These evaluations used the approved dose of aztreonam alone (2 g every 6–8 h) for severe infections in patients with normal renal function [28, 29] and avibactam doses supported by preclinical experiments to determine PK-PD targets, which used a human-simulated dose of 2000 mg aztreonam combined with 375 or 600 mg of avibactam [12, 13]. In Part B Cohorts 1 and 2, aztreonam-avibactam doses of 2000/375 mg and 2000/600 mg 1-h infusions every 6 h (q6h) were evaluated. Potentially clinically significant plasma aspartate transaminase (AST) and alanine transaminase (ALT) elevations prompted the early termination of enrolment in Part B Cohort 2. In Part B Cohort 3, the aztreonam dose was reduced to 1500 mg with avibactam 600 mg administered as 2-h infusions q6h. Although stopping criteria were not met, the sponsor temporarily stopped enrolment again due to transaminase elevations.
Following these clinical observations, early population PK models (designated Iteration 1) were developed. For aztreonam, the Iteration 1 model utilized interim PK data from the Phase I study supplemented with published data on aztreonam alone [32, 33]. For avibactam, the Iteration 1 model included data from the ceftazidime-avibactam program [21, 22] in addition to the Phase I PK data. Exposure and joint PTA simulations were performed from the Iteration 1 model to support selection of reduced doses for Phase I Part B Cohorts 4 and 5, and Part C. Since all of the aztreonam PK data included in the model was derived from only healthy subjects, various aztreonam “patient-like” PK assumptions were explored in these simulations (Supplementary Table 1). The final regimen evaluated in Phase I Part C comprised an aztreonam-avibactam loading dose of 500/137 mg (30-min infusion) immediately followed by an extended loading dose with subsequent maintenance doses of 1500/410 mg (3-h infusions) q6h; this regimen was also evaluated (with a minor modification) in Phase IIa Cohort 1 [10].
During enrolment in Phase IIa Cohort 1, the aztreonam and avibactam population PK models were updated (Iteration 2), which included final data from the above Phase 1 trial. For aztreonam, the Iteration 1 model contained additional published PK data in healthy subjects and subjects with renal impairment, but did not include data from patients with cIAI or cUTI. Thus for Iteration 2, variances and covariates for aztreonam were imputed based on the established avibactam models developed during the ceftazidime-avibactam clinical program, which incorporated data from Phase II and Phase III patients. While these variances and covariates are expected to differ between the two drugs, this approach was considered more realistic than relying solely on aztreonam PK variability in healthy volunteers. As both drugs share a primary elimination pathway, it was reasonable to include for aztreonam assumptions based on variability in avibactam PK, which has been well explored in the target patient population. From the Iteration 2 model, additional simulations were conducted to support dose regimen selection for Phase IIa (Cohorts 2 and 3) and for subsequent Phase III evaluation. These simulations also included adjustments for patients with moderate and severe renal impairment.
Data sources
Data sources, including numbers of PK samples, and key objectives and assumptions used for the two aztreonam and avibactam population PK model iterations are shown in Table 2. The aztreonam population PK models used subject PK data from the Phase I aztreonam-avibactam trial [9] and two published studies of aztreonam in healthy subjects and subjects with renal impairment [32, 33]. Both avibactam model iterations used PK data obtained during the ceftazidime-avibactam clinical program, including healthy and renally impaired subjects and patients with cIAI (and cUTI in Iteration 2) [20,21,22]. The Phase I trials (of avibactam ± aztreonam or avibactam ± ceftazidime) included intensive blood sampling for PK analysis (up to 17 samples per subject per day), and the Phase II and III trials (of ceftazidime-avibactam) included sparse PK sampling protocols (up to four samples per patient per day) for blood collection mainly on Day 3. In the aztreonam renal impairment studies, 10–12 PK samples per subject were obtained over 24–48 h [32, 33]. Baseline and demographic characteristics of subjects in each model are summarized in Table 3.
Bioanalytical methods
For subject PK data from the aztreonam-avibactam and ceftazidime-avibactam clinical programs, plasma samples were assayed by Covance Bioanalytical Laboratory, Harrogate, UK, using validated liquid chromatographic-tandem mass spectrometric (LC–MS/MS) methods. The lower limits of quantification were 0.1 mg/L for aztreonam and 0.01 mg/L for avibactam. The plasma concentration data for aztreonam taken from literature used a microbiological agar diffusion method with Escherichia coli (test organism) with a lower limit of quantification of 0.04 mg/L [32, 33].
Modeling software
Population PK modeling used the FOCE-I method in NONMEM v7.2.0 or higher (Icon Development Solutions, Ellicott City, MD, USA). Simulations were performed with NONMEM and/or R v3.3.0 or higher (https://www.r-project.org/).
Population PK model development
Aztreonam
A two-compartment model (Supplementary Table 2) was fitted to interim data from 27 healthy volunteers from the Phase I aztreonam-avibactam trial (Iteration 1a) and subsequently a total of 69 subjects from both the Phase I trial and two published studies (Iteration 1b), to include the effect of renal impairment. Renal impairment was modeled with a power model for CrCL on clearance (CL).
For model Iteration 2, the two-compartment disposition model was fitted to data from 107 healthy subjects and subjects with renal impairment; all parameters used standard allometry (exponents of 1 for volumes and 0.75 for CL and inter-compartmental clearance [Q] respectively; Supplementary Table 3). Covariate effects of CrCL on CL (two-part linear functions with steeper slope for CrCL < 80 mL/min) and age (> 50 or > 65 years) on apparent volume of the central compartment (Vc) and apparent volume of the peripheral compartment (Vp), and nonlinearity of aztreonam protein binding (Bmax, B50) were included.
Avibactam
For model Iteration 1, a two-compartment model was fitted to data from 315 healthy subjects, subjects with renal impairment and patients with cIAI, with covariate effects for CrCL on CL and for cIAI patients on both CL (45% increase) and Vc (166% increase) compared with healthy subjects (Supplementary Table 4).
For model Iteration 2, an updated two-compartment model was fitted to data from 1836 healthy subjects, subjects with renal impairment, and patients with cIAI or cUTI from the ceftazidime-avibactam development program (Supplementary Table 5). The model no longer included an adjustment for CL as seen for Phase II cIAI patients; Phase III cIAI patient effect on Vc remained (27.5% increase) but was smaller than that for Phase II patients with cIAI.
Exposure and PTA analyses
The two sets of population PK model iterations were used to simulate exposures and joint PTA for various aztreonam-avibactam dose regimens; simulations based on Iteration 1 explored the impact of different scenarios for patient covariate effects as described below, and those based on Iteration 2 included evaluations of the impact of renal function (normal, mild, moderate, and severe renal impairment). Exposures were calculated from total plasma concentrations, and PTA simulations used free (unbound) fractions for aztreonam and avibactam of 58% [18] and 92%, respectively [30].
PK-PD targets
In vitro global surveillance studies have reported that > 99% of clinically relevant Enterobacterales isolates, including MBL producers, are inhibited at aztreonam-avibactam MICs ≤ 8 mg/L (with avibactam fixed at 4 mg/L) [34,35,36,37,38]; ≤ 8 mg/L is also the tentative susceptible MIC breakpoint against target Gram-negative bacteria for the aztreonam-avibactam combination. Plasma PK-PD targets for the aztreonam and avibactam combination against MBL- and extended-spectrum β-lactamase (ESBL)-producing clinical Enterobacterales isolates have been derived from an in vitro hollow fiber infection model (HFIM) [13] with validation of the avibactam target in an in vivo mouse thigh model [12]; a 60% fT > MIC value for aztreonam was the exposure measure most closely associated with efficacy [25]. Experiments using fixed doses of aztreonam in these models identified a CT for avibactam of 2.5 mg/L; a target of 50% fT was within the range of efficacy estimates for the HFIM [13] and was considered conservative compared with the murine model (maximal effect of avibactam [combined with aztreonam, with aztreonam exposure fixed at > 50% fT > MIC] against MBL- and ESBL-producing E. coli and Klebsiella pneumoniae isolates where efficacy was achieved at 35–40% fT = 2.0–2.5 mg/L) [13]. Based on these studies, a joint PK-PD target (defined as attainment of 60% fT > MIC for aztreonam, and 50% fT > 2.5 mg/L for avibactam, achieved simultaneously) was evaluated in the current PTA analyses, with MIC = 8 mg/L considered as the primary target.
Iteration 1 simulations for Phase I dose modification and Phase IIa dose selection
Simulations using Iteration 1 population PK aztreonam and avibactam models (Supplementary Tables 2 and 4, respectively) evaluated various aztreonam-avibactam dose regimens (1000 subjects per simulation), including extended 3-h infusions and 6-h (continuous) infusions q6h, and loading doses in subjects with normal renal function. The aim of these simulations was to select a dose regimen that provided adequate exposures and joint PTA ≥ 90% at MIC = 8 mg/L while attempting to reduce the frequency of transaminase elevations observed in earlier cohorts of the Phase I trial [9]. Since the only available aztreonam PK data were from healthy subjects, five scenarios (designated Cases 1–5; Supplementary Table 1) encompassing additional assumptions on the influences on patient PK on key PK parameters were explored. This included using the CL value for patients with cystic fibrosis (Cases 2 and 3). Patients with cystic fibrosis have been reported to have increased aztreonam CL values, as well as increased variability, relative to healthy individuals [18]. In Cases 4 and 5, alterations in CL and Vc compared to healthy volunteers and associated variances observed for avibactam and ceftazidime in cIAI patients were applied to aztreonam [20].
Iteration 2 simulations for Phase IIa dose optimization and Phase III dose selection
The Iteration 2 population PK aztreonam and avibactam models (Supplementary Tables 3 and 5, respectively) were used to simulate exposure and joint PTA for various aztreonam-avibactam dose regimens in patients with cIAI with normal renal function and mild, moderate, or severe renal impairment (estimated CrCL > 80 mL/min, > 50 to ≤ 80 mL/min, > 30 to ≤ 50 mL/min, and > 15 to ≤ 30 mL/min, respectively). The aim was to optimize doses for Phase IIa and Phase III patients with normal renal function, and to select regimens for patients with moderate and severe renal impairment. In the absence of aztreonam patient PK data, the aztreonam simulation model was adapted from that shown in Supplementary Table 3 to include an increase of Vc for patients with cIAI of 27.5% (based on avibactam data from Phase III patients with cIAI), with no change to CL. Aztreonam parameter covariance was assumed to be the same as for the Iteration 2 avibactam model, leading to a greater dispersion across patients.
The avibactam simulation model [21] used Phase III cIAI parameters for Vc with the major covariate of CrCL on CL. Covariates of age, weight, and CrCL from Phase III patients in the ceftazidime-avibactam program [22] were used to create multivariate covariate distributions. Exposure and joint PTA simulations were conducted for 5000 simulated patients for each renal function group and dose regimen by resampling these distributions. For simulations of patients with moderate and severe renal impairment, CrCL values were assumed to follow a uniform distribution within the designated range for each category.
Dose regimen selection for patients with normal renal function or mild renal impairment was based on achieving ≥ 90% joint PTA in simulated patients with cIAI based on the above PK-PD targets and consideration of the safety profile. A variety of dose regimens for potential clinical evaluation in patients with moderate and severe renal impairment, maintaining a fixed aztreonam to avibactam dose ratio across renal function groups, were evaluated using the following criteria: (1) matching daily aztreonam area under the plasma concentration curve (AUC(0–24)) to the normal renal function group (without avibactam exposure exceeding that in the mild renal impairment group); and (2) joint PTA ≥ 90% at MIC = 8 mg/L, taking into account the differential effects of renal impairment on aztreonam and avibactam (matching AUC(0–24) for aztreonam to normal renal function would implicitly result in exceeding the corresponding avibactam exposures in normal renal function; this was deemed acceptable given the known safety profile of avibactam).
Results
Exposure and PTA analyses for Phase I dose modification and Phase IIa dose selection
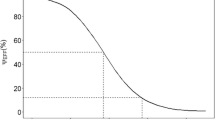
Simulations based on the Iteration 1 population PK models were used to select aztreonam-avibactam maintenance dose regimens for Phase I Part B Cohorts 4 and 5 and Part C [9], with the aim of achieving adequate PTA with reduced doses and increased infusion durations. For PK assumptions based on healthy subjects (Case 1; see Supplementary Table 1), aztreonam-avibactam 1500/450 mg maintenance 3-h infusions q6h achieved joint PTA > 90% at MICs ≤ 16 mg/L; with ‘patient-like’ aztreonam PK assumptions (Cases 2–5), joint PTA was > 90% for MICs ≤ 8 mg/L (Fig. 1A). Of note, achievement of joint PTA ≥ 90% for MICs < 8 mg/L in these scenarios was driven primarily by the avibactam dose, with avibactam maintenance doses > 400 mg q6h needed for Cases 2–5 (data not shown). Based on these analyses, an aztreonam-avibactam maintenance dose regimen of 1500/450 mg 3-h infusions q6h was selected for Part B Cohort 4. Case 4 (cIAI patient-like) PK assumptions for aztreonam resulted in the most variability (and therefore the lowest joint PTA), and Case 4 was therefore the main focus in subsequent simulations.
Joint PTA by MIC at steady-state for selected aztreonam-avibactam dose regimens in simulated subjects with normal renal functiona. A Aztreonam-avibactam 1500/450 mg maintenance doses (3-h infusions q6h): Impact of aztreonam PK scenarios (aztreonam PK model Iteration 1a / avibactam PK model Iteration 1), overlaid with aztreonam-avibactam MIC distribution against MBL-producing Enterobacterales collected during 2016–2020 as part of the ATLAS Global Surveillance Programb. B Potential aztreonam-avibactam maintenance doses: 3-h infusions q6h vs continuous infusions (aztreonam PK model Iteration 1b / avibactam PK model Iteration 1)c. C Joint PTA during the first 6-h dosing interval on day 1 for aztreonam-avibactam 1500/410 mg q6h (continuous infusions) with and without loading dose 500/137 mg (aztreonam PK model Iteration 1b / avibactam PK model Iteration 1)c. D Potential aztreonam-avibactam maintenance doses (3-h infusions q6h): joint PTA with varying aztreonam and avibactam doses (aztreonam PK model Iteration 2 / avibactam PK model Iteration 2)d. CI continuous infusions, cIAI complicated intra-abdominal infection, CL clearance, IIV inter-individual variability, MIC minimum inhibitory concentration, PTA, probability of target attainment, q6h, every 6 h, Vc apparent volume of the central compartment. aProbability of attaining the joint PK-PD target, defined as free plasma concentrations of aztreonam above the specified MIC for 60% of each dosing interval (60% fT > MIC mg/L) and free concentrations of avibactam above 2.5 mg/L for 50% of each dosing interval (50% fT > 2.5 mg/L). Both targets needed to be achieved simultaneously to meet the joint target. Joint PTA at steady-state is reported unless stated otherwise. bAztreonam PK scenarios (Supplementary Table 1): Case 1, healthy subjects’ CL and IIV (5.5 L/h and 8.8%, respectively; Supplementary Table 1); Case 2, CL for patients with cystic fibrosis (6.1 L/h) [18] and IIV of 17.6% (i.e., doubled from healthy subjects in Case 1); Case 3, CL for patients with cystic fibrosis (6.1 L/h) [18] and IIV of 27.9% (equal to that of avibactam; Supplementary Table 3); Case 4, 45% increase in CL and 166% increase in Vc, as observed in Phase II patients with cIAI for avibactam; IIVs as for avibactam (27.9% and 32.1%; Supplementary Table 3); and Case 5, 23% increase in CL and 108% increase in Vc, as observed in patients with cIAI for ceftazidime; respective IIVs of 37.4% and 49.0% from the early ceftazidime PK model [20]. cAztreonam PK scenario Case 4 (Supplementary Table 1). dSimulations included a loading dose of one-third of the maintenance dose given by 30-min infusion. MIC distributions reproduced with permission [37]
Following completion of Part B Cohort 4, simulations explored reductions in the aztreonam and/or avibactam component of the combined aztreonam-avibactam dose, and comparing 3-h infusions with continuous infusions, to select a dose regimen for Part B Cohort 5. In these analyses, achievement of joint PTA at MIC = 8 mg/L was driven primarily by the avibactam dose, with no meaningful differences in joint PTA for 3-h infusions q6h compared with continuous infusions at steady-state (Fig. 1B). It was apparent from these simulations that addition of a 30-min infusion loading dose of aztreonam-avibactam 500/137 mg was required to improve joint PTA during the first 6 h for continuous infusion (Fig. 1C). An aztreonam-avibactam maintenance dose regimen of 1500/410 mg 3-h infusions q6h was selected for Part B Cohort 5, with loading doses of 500/137 mg 30-min infusion, immediately followed by a 1500/410 mg 2.5-h infusion (extended loading dose), followed by 1500/410 mg 3-h infusions q6h (maintenance dose). This regimen was further evaluated in Phase I Part C. This final Phase I dose regimen was adapted for evaluation in cIAI patients with estimated CrCL > 50 mL/min in Phase IIa Cohort 1 to give the extended loading dose by 3-h infusion, a minor change for clinical acceptability with no meaningful impact on exposure or joint PTA.
Exposure and PTA analyses for Phase IIa dose optimization and Phase III dose selection
During enrolment in Cohort 1 of the Phase IIa study of aztreonam-avibactam in patients with cIAI [10], additional simulations were performed using Iteration 2 population PK models to select doses for evaluation in Phase IIa Cohorts 2 and 3 and for the Phase III trials, including doses for patients with moderate and severe renal impairment. Predicted steady-state exposures and joint PTA at MIC = 8 mg/L for the selected dose regimens are shown in Table 4.
Predictions based on Iteration 2 simulations found that joint PTA at MIC = 8 mg/L at steady-state was slightly lower (87%) for patients with normal renal function (estimated CrCL > 80 mL/min) receiving the Phase IIa Cohort 1 aztreonam-avibactam dose regimen compared with the Iteration 1 simulations (Table 4 and Fig. 1D). Patients with mild renal impairment (estimated CrCL > 50 to ≤ 80 mL/min) receiving the same doses had higher predicted aztreonam and avibactam exposures (steady-state AUC(0–24) ratios of 1.40 for and 1.39, respectively) and joint PTA > 95% (Table 4). However, joint PTA > 90% for patients with normal renal function could be achieved by increasing the aztreonam-avibactam loading dose to 500/167 mg and extended loading and maintenance doses to 1500/500 mg (Table 4 and Fig. 1D). Accordingly, this increased dose regimen was assessed in patients with estimated CrCL > 50 mL/min in Phase IIa Cohort 2, and expanded into Phase IIa Cohort 3 following favorable safety and tolerability assessments for the first 10 patients enrolled in each of Cohorts 1 and 2 [10].
The exposure and PTA results for the aztreonam-avibactam loading and maintenance dose regimen selected for Phase IIa Cohorts 2 and 3 above (designated the ‘reference dose’) for patients with estimated CrCL > 50 mL/min were used as reference points for selecting dose regimens for patients with moderate and severe renal impairment (estimated CrCL > 30 to ≤ 50 mL/min and > 15 to ≤ 30 mL/min, respectively). Simulations for patients with moderate renal impairment evaluated aztreonam-avibactam maintenance doses of 45–70% of the daily 'reference dose' (maintaining a 3:1 ratio) administered by 3-h infusion q6h with either proportionate loading and extended loading doses, or with the higher ‘reference’ loading (500/167 mg [30-min infusion]) and extended loading doses (1500/500 mg [3-h infusion]). The aztreonam-avibactam maintenance dose for patients with moderate renal impairment that best matched exposures in patients with normal renal function was 750/250 mg q6h, giving steady-state AUC(0–24) ratios of 0.95 for aztreonam and 1.14 for avibactam and joint PTA at MIC = 8 mg/L of 93.1% (Table 4). A proportionate loading dose of 250/83 mg and an extended loading dose of 750/250 mg gave joint PTA of approximately 74% in the first 6-h dosing period. Using the higher ‘reference’ loading (500/167 mg) and extended loading (1500/500 mg) doses for patients with moderate renal impairment resulted in joint PTA at MIC = 8 mg/L > 90% during the first 6-h dosing interval, albeit with transient increases in exposures relative to patients with normal renal function receiving the 'reference dose'. This regimen was therefore selected for patients with moderate renal impairment in the Phase IIa (Cohorts 2 and 3) and Phase III aztreonam-avibactam trials.
Simulations for patients with severe renal impairment evaluated aztreonam-avibactam maintenance doses of 30–40% of the daily ‘reference dose’ administered q6h, every 8 h (q8h) or every 12 h with proportionate loading and extended loading doses. A regimen comprising a loading dose of a 675/225 mg 30-min infusion, an extended loading dose of 675/225 mg 3-h infusion, and maintenance doses of 675/225 mg 3-h infusions q8h, giving steady-state AUC(0–24) ratios of 0.88 for aztreonam and 1.47 for avibactam and joint PTA at MIC = 8 mg/L of 91.6% (Table 4), was selected for evaluation in the Phase III trials. A loading dose of 675/225 mg 30-min infusion followed immediately by an extended loading dose of 675/225 mg 3-h infusion for patients with severe renal impairment resulted in joint PTA at MIC = 8 mg/L of 90.6% during the first 8-h dosing interval, again with transient increases in exposures relative to the ‘reference dose’.
Discussion
Dose regimen selection for the aztreonam-avibactam clinical program has been guided by approved dosing recommendations for aztreonam monotherapy, target pathogen MIC distributions for aztreonam and avibactam in combination, and an iterative process of population PK modeling and PTA simulations evaluating a range of dosing scenarios. A similar process of iterative population PK modeling was undertaken to support dose modification in order to ensure adequate dosing for patients with renal impairment in the ceftazidime-avibactam clinical program [24].
Aztreonam as a monotherapy is approved at doses up to 8 g/day, and is associated with elevations of plasma AST and ALT levels in some patients; such transaminase elevations are typically transient and asymptomatic, and normalize during treatment or following treatment discontinuation [39, 40]. In the Phase I aztreonam-avibactam dose-finding trial, various dose regimens were explored, including monitoring of transaminase effects, facilitated by a three-part study design with sequential cohorts of healthy subjects [9]. The analyses described here, which supported the selection and modification/optimization of aztreonam-avibactam dosages for clinical investigation, demonstrate the following: (1) aztreonam-avibactam extended (3-h) infusions and q6h dosing in most patients are optimal to achieve adequate exposures/joint PTA; (2) when accounting for patient variability, avibactam dose is the limiting factor for achieving the joint PTA target at MIC < 8 mg/L; and (3) a loading dose improves joint PTA in the first dosing interval. These analyses benefited from a large and robust population PK dataset for avibactam, including patients with cIAI and cUTI from the ceftazidime-avibactam clinical development program in Iteration 2 [21, 22]. In contrast, no large-scale population PK models for aztreonam in patients with complicated infections were available. Accordingly, for the simulations based on PK model Iteration 1, various assumptions were tested to make the aztreonam data more representative of infected patients, and for those based on PK model Iteration 2, scaling of Vc at steady state was assumed to be the same as that of avibactam in Phase III patients with cIAI (i.e., 27.5% higher). While a non-linear protein binding function for the aztreonam Iteration 2 population PK models provided the best fit to the data, concentration-independent binding of aztreonam to human plasma proteins has been reported [16] and confirmed in vitro over the range of clinically relevant concentrations (unpublished data). An alternative model (not presented) without non-linearity in the observed concentrations provided very similar CL and Vc parameter estimates. As such, more current modeling iterations use the simplified aztreonam PK model.
Exposure and joint PTA simulations were conducted using Iteration 1 PK models part way through the Phase I aztreonam-avibactam trial to support downward dose adjustments with the aim of achieving adequate PTA while reducing the potential for observed transaminase elevations. These analyses suggested that reduced aztreonam maintenance doses with extended (3-h) infusions administered q6h resulted in adequate joint PTA for the primary target (60% fT > 8 mg/L for aztreonam and 50% fT > 2.5 mg/L for avibactam); when renal impairment was considered, inclusion of a loading dose resulted in more simulated patients achieving the joint PK-PD target during the initial dosing interval. For the simulations based on the Iteration 2 PK models, scaling on Vc and variability of aztreonam CL and Vc used values from avibactam PK model Iteration 2, which included Phase III patients with cIAI or cUTI. The additional data allowed variability of avibactam in the patient population to be more robustly predicted; joint PTA at the target of MIC = 8 mg/L for the Phase IIa Cohort 1 dose regimen (loading 500/137 mg, maintenance 1500/410 mg q6h) in simulated patients with normal renal function was slightly lower than previously estimated based on the Iteration 1 simulations. The increased variability is the likely reason that Iteration 2 simulations models required an increase in avibactam dose to achieve joint PTA > 90%. Accordingly, increased loading (500/167 mg) and maintenance (1500/500 mg q6h) doses were selected for evaluation in Phase IIa Cohorts 2 and 3 and for the Phase III trials. Simulations for moderate and severe renal impairment using the Iteration 2 PK models also highlighted the importance of the loading dose for attaining joint PTA > 90% at MIC = 8 mg/L during the initial dosing interval (74% vs > 95% for a proportionate loading dose vs the 'reference' loading dose in patients with moderate renal impairment). Of note, aztreonam monotherapy doses are not adjusted for moderate renal impairment [28, 29]. Considering the differential renal clearance of the two drugs and the fixed 3:1 aztreonam to avibactam combination, the use of loading doses in patients with moderate or severe renal impairment to support achievement of adequate aztreonam exposures will inevitably produce transient increases in avibactam exposures beyond those predicted for patients with normal renal function. However, estimated avibactam exposures for the selected aztreonam-avibactam dose regimens for moderate or severe renal impairment are of similar magnitude to those in subjects with mild renal impairment and are thus considered unlikely to result in additional safety/tolerability risks.
Strengths of the current analyses include the large patient PK database for avibactam, and use of a simultaneous joint PTA target, which is more conservative than approaches used for some other antibiotic/inhibitor combinations [41, 42]. The use of Phase III cIAI avibactam data and assumptions from the ceftazidime-avibactam program to support the development of aztreonam-avibactam is justified by the lack of drug–drug interactions between ceftazidime and avibactam [43, 44] as well as the absence of interactions between aztreonam and avibactam demonstrated in the Phase I dose-finding trial [9]. The lack of patient PK data for aztreonam informing the model (particularly in respect of the critically ill patient population), and the use of a combination of (older) published PK data for subjects with renal impairment [32, 33] and (more recent) data from the Phase I trial (with different analytical methods for measuring aztreonam plasma concentrations) are nevertheless potential limitations of the current analyses. However, aztreonam PK data obtained from the literature also included some subjects with normal renal function [32, 33]; while not formally evaluated, the plasma concentrations were consistent with our Phase I observations in healthy subjects (data not shown). The absence of data for patients with severe hypoalbuminemia (nephrotic syndrome or other proteinuric diseases) limits the clinical applicability of these simulations. Moreover, models and PK simulations were based on calculation of free concentrations using a fixed protein-binding estimate for joint PTA calculations. Higher unbound drug fractions due to hypoalbuminemia could be expected to increase V and CL [45, 46].
Subsequent to the current analyses, a simultaneous population PK model for aztreonam and avibactam has been developed to support initial dose selection for pediatric clinical studies [47]. Future model iterations will include aztreonam plasma PK data from patients in the Phase IIa and Phase III aztreonam-avibactam clinical trials, and from a Phase I trial of aztreonam-avibactam in subjects with severe renal impairment (NCT04486625) and utilize simultaneous modeling to efficiently estimate correlations of PK variability between aztreonam and avibactam.
Given the severely limited available treatment options for infections caused by MBL-producing Gram-negative bacteria, there have been reports on the use of combinations of ceftazidime-avibactam plus aztreonam (frequently combined with other antibiotics) in severely ill hospitalized patients, with some evidence of effectiveness in single patient cases and small case series [48,49,50]. Based on these data, ceftazidime-avibactam plus aztreonam has been included in recent international treatment guidelines [51,52,53]; guidance from the Infectious Diseases Society of America (IDSA) includes a suggested dose regimen for ceftazidime-avibactam 2.5 g plus aztreonam 2 g by 3-h infusions q8h or q6h (q6h dosing is preferred if possible) [52,53,54]. While it is encouraging that positive outcomes have been reported for ceftazidime-avibactam plus aztreonam, the IDSA recommendation does not include a loading dose, and based on the current simulations, the proposed regimen would be unable to achieve sufficient avibactam exposures to ensure > 90% joint PTA for aztreonam-avibactam at MIC = 8 mg/L. As such there is a possibility of inadequate PK-PD target attainment (particularly at higher-pathogen MICs) for ceftazidime-avibactam plus aztreonam dosed according to the IDSA recommendation. Also, without loading doses, there will be on average a longer time to achieve targets in the critical early phase of treatment. In addition, the safety (including transaminase liability) of ceftazidime-avibactam plus aztreonam is unknown, although transaminase elevations have been reported to be more frequent with combinations including 8 g/day of aztreonam compared to those including 6 g/day [55]. Overall, the “off-label” use of combinations of ceftazidime-avibactam plus aztreonam might not provide adequate exposures for all patients across the range of target pathogen susceptibilities.
In summary, results from two influential iterations of population PK modeling and simulations for efficacy (based on joint PTA analyses) during and after completion of the aztreonam-avibactam Phase I dose-finding trial have supported selection and modification of aztreonam-avibactam dosage regimens (based on a 3:1 fixed ratio) for Phase IIa and Phase III clinical evaluation. The final selected aztreonam-avibactam dose regimen for Phase III patients with estimated CrCL > 50 mL/min is a 500/167 mg loading 30-min infusion followed immediately by 1500/500 mg maintenance 3-h infusions q6h. Adjustments for moderate renal impairment (estimated CrCL > 30 to ≤ 50 mL/min) and severe renal impairment (> 15 to ≤ 30 mL/min) account for the differential renal clearance of aztreonam and avibactam and share a consistent schedule of loading doses (30-min infusions) followed by extended loading doses and maintenance doses (3-h infusions) q6h or q8h, respectively.
Data availability
Upon request, and subject to certain criteria, conditions, and exceptions, see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information. Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
Mojica MF, Rossi MA, Vila AJ, Bonomo RA (2022) The urgent need for metallo-beta-lactamase inhibitors: an unattended global threat. Lancet Infect Dis 22:e28–e34. https://doi.org/10.1016/S1473-3099(20)30868-9
Bush K, Bradford PA (2020) Epidemiology of beta-lactamase-producing pathogens. Clin Microbiol Rev 33:e00047-e119. https://doi.org/10.1128/CMR.00047-19
Centers for Disease Control and Prevention (2019) Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 10 Jun 2021
Karaiskos I, Galani I, Souli M, Giamarellou H (2019) Novel β-lactam-β-lactamase inhibitor combinations: expectations for the treatment of carbapenem-resistant Gram-negative pathogens. Expert Opin Drug Metab Toxicol 15:133–149. https://doi.org/10.1080/17425255.2019.1563071
Das S, Li J, Riccobene T, Carrothers TJ, Newell P, Melnick D, Critchley IA, Stone GG, Nichols WW (2019) Dose selection and validation for ceftazidime-avibactam in adults with complicated intra-abdominal infections, complicated urinary tract infections, and nosocomial pneumonia. Antimicrob Agents Chemother 63:e02187-e12118. https://doi.org/10.1128/AAC.02187-18
Abboud MI, Damblon C, Brem J, Smargiasso N, Mercuri P, Gilbert B, Rydzik AM, Claridge TD, Schofield CJ, Frere JM (2016) Interaction of avibactam with class b metallo-beta-lactamases. Antimicrob Agents Chemother 60:5655–5662. https://doi.org/10.1128/AAC.00897-16
Daikos GL, da Cunha CA, Rossolini GM, Stone GG, Baillon-Plot N, Tawadrous M, Irani P (2021) Review of ceftazidime-avibactam for the treatment of infections caused by Pseudomonas aeruginosa. Antibiotics (Basel, Switzerland) 10:1126. https://doi.org/10.3390/antibiotics10091126
Soriano A, Carmeli Y, Omrani AS, Moore LSP, Tawadrous M, Irani P (2021) Ceftazidime-avibactam for the treatment of serious gram-negative infections with limited treatment options: a systematic literature review. Infect Dis Ther 10:1989–2034. https://doi.org/10.1007/s40121-021-00507-6
Edeki T, Zhou D, van den Berg F, Broadhurst H, Holmes WC, Peters G, Sunzel M, O'Brien S (2016) A phase I, 3-part placebo-controlled randomised trial to evaluate the safety, tolerability and pharmacokinetics of aztreonam-avibactam in healthy subjects. Presented at: 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). Amsterdam, the Netherlands
Cornely OA, Cisneros JM, Torre-Cisneros J, Rodriguez-Hernandez MJ, Tallon-Aguilar L, Calbo E, Horcajada JP, Queckenberg C, Zettelmeyer U, Arenz D, Rosso-Fernandez CM, Jimenez-Jorge S, Turner G, Raber S, O’Brien S, Luckey A, COMBACTE-CARE consortium/REJUVENATE Study Group (2020) Pharmacokinetics and safety of aztreonam/avibactam for the treatment of complicated intra-abdominal infections in hospitalized adults: results from the REJUVENATE study. J Antimicrob Chemother 75:618–627. https://doi.org/10.1093/jac/dkz497
Carmeli Y, Cisneros Herreros JM, Paul M, Daikos G, Wang M, de La Torre Cisneros J, Singer G, Titov I, Gumenchuk I, Zhano Y, Jiménez Rodríguez R-M, Liang L, Chen G, Pyptiuk O., Aksoy F., Rogers H, Wible M, Arhin F, Luckey A, Leaney J, Pijpstra R, Chow JW (2023) Efficacy and safety of aztreonam-avibactam for the treatment of serious infections due to gram-negative bacteria, including metallo-β-lactamase-producing pathogens: phase 3 REVISIT study. 2893 A. Presented at: IDWeek 2023. Boston, MA, USA
Crandon JL, Nicolau DP (2013) Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-beta-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. https://doi.org/10.1128/AAC.01989-12
Singh R, Kim A, Tanudra MA, Harris JJ, McLaughlin RE, Patey S, O’Donnell JP, Bradford PA, Eakin AE (2015) Pharmacokinetics/pharmacodynamics of a beta-lactam and beta-lactamase inhibitor combination: a novel approach for aztreonam/avibactam. J Antimicrob Chemother 70:2618–2626. https://doi.org/10.1093/jac/dkv132
Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N (2011) Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. https://doi.org/10.1128/AAC.00756-10
Li H, Estabrook M, Jacoby GA, Nichols WW, Testa RT, Bush K (2015) In vitro susceptibility of characterized beta-lactamase-producing strains tested with avibactam combinations. Antimicrob Agents Chemother 59:1789–1793. https://doi.org/10.1128/AAC.04191-14
Mattie H (1988) Clinical pharmacokinetics of aztreonam. Clin Pharmacokinet 14:148–155. https://doi.org/10.2165/00003088-198814030-00003
Mattie H (1994) Clinical pharmacokinetics of aztreonam. An update Clin Pharmacokinet 26:99–106. https://doi.org/10.2165/00003088-199426020-00003
Vinks AA, van Rossem RN, Mathot RA, Heijerman HG, Mouton JW (2007) Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using monte carlo simulation. Antimicrob Agents Chemother 51:3049–3055. https://doi.org/10.1128/aac.01522-06
Xu H, Zhou W, Zhou D, Li J, Al-Huniti N (2017) Evaluation of aztreonam dosing regimens in patients with normal and impaired renal function: a population pharmacokinetic modeling and Monte Carlo simulation analysis. J Clin Pharmacol 57:336–344. https://doi.org/10.1002/jcph.810
Li J, Knebel W, Riggs M, Zhou D, Nichols WW, Das S (2012) Population pharmacokinetic modelling of ceftazidime (CAZ) and avibactam (AVI) in healthy volunteers and patients with complicated intra-abdominal infection (cIAI). A-634. Presented at: 52nd Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC). San Francisco, CA, USA
Li J, Zhou D, Das S, Lovern M, Green M, Chiu J, Riccobene T, Carrothers T, Al-Huniti N (2015) Population PK Modeling for ceftazidime-avibactam (CAZ-AVI) in patients with complicated intra-abdominal infection (cIAI) and complicated urinary tract infection (cUTI). Presented at: American Association of Pharmaceutical Scientists Annual Meeting and Exposition. Orlando, FL, USA
Li J, Nichols WW, Zhou D, Das S (2015) Population pharmacokinetic modeling of ceftazidime and avibactam and probability of target attainment to support the dosing regimen in patients with nosocomial pneumonia including ventilator-associated pneumonia. Presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). Copenhagen, Denmark
Li J, Lovern M, Green ML, Chiu J, Zhou D, Comisar C, Xiong Y, Hing J, MacPherson M, Wright JG, Riccobene T, Carrothers TJ, Das S (2019) Ceftazidime-avibactam population pharmacokinetic modeling and pharmacodynamic target attainment across adult indications and patient subgroups. Clin Transl Sci 12:151–163. https://doi.org/10.1111/cts.12585
Li J, Lovern M, Riccobene T, Carrothers TJ, Newell P, Das S, Talley AK, Tawadrous M (2020) Considerations in the selection of renal dosage adjustments for patients with serious infections and lessons learned from the development of ceftazidime-avibactam. Antimicrob Agents Chemother 64:e02105-02119. https://doi.org/10.1128/AAC.02105-19
Nichols WW, Newell P, Critchley IA, Riccobene T, Das S (2018) Avibactam pharmacokinetic/pharmacodynamic targets. Antimicrob Agents Chemother 62:e02446-e12417. https://doi.org/10.1128/AAC.02446-17
Swabb EA, Sugerman AA, McKinstry DN (1983) Multiple-dose pharmacokinetics of the monobactam azthreonam (SQ 26,776) in healthy subjects. Antimicrob Agents Chemother 23:125–132. https://doi.org/10.1128/AAC.23.1.125
Swabb EA, Sugerman AA, Platt TB, Pilkiewicz FG, Frantz M (1982) Single-dose pharmacokinetics of the monobactam azthreonam (SQ 26,776) in healthy subjects. Antimicrob Agents Chemother 21:944–949. https://doi.org/10.1128/AAC.21.6.944
Bristol-Myers Squibb Co. (2018) AZACTAM® (aztreonam injection): prescribing information. http://packageinserts.bms.com/pi/pi_azactam.pdf. Accessed 19 Jul 2022
Bristol-Myers Squibb Pharmaceuticals Ltd. (2021) Azactam 1g powder for solution for injection or infusion, vial. Summary of product characteristics. https://www.medicines.org.uk/emc/product/3773/smpc. Accessed 19 Jul 2022
Pfizer (2022) Summary of product characteristics: Zavicefta 2 g/0.5 g powder for concentrate for solution for infusion. https://www.ema.europa.eu/documents/product-information/zavicefta-epar-product-information_en.pdf. Accessed 7 Sept 2022
Allergan (2022) AVYCAZ (ceftazidime and avibactam) for injection, for intravenous use. https://www.rxabbvie.com/pdf/avycaz_pi.pdf
Mihindu JC, Scheld WM, Bolton ND, Spyker DA, Swabb EA, Bolton WK (1983) Pharmacokinetics of aztreonam in patients with various degrees of renal dysfunction. Antimicrob Agents Chemother 24:252–261. https://doi.org/10.1128/AAC.24.2.252
el Guinaidy MA, Nawishy S, Abd el Bary M, Sabbour MS (1989) Single-dose pharmacokinetics of aztreonam in healthy volunteers and renal failure patients. J Chemother 1:164–169. https://doi.org/10.1080/1120009x.1989.11738886
Sader HS, Mendes RE, Arends SJR, Carvalhaes CG, Castanheira M (2022) Antimicrobial activities of aztreonam-avibactam and comparator agents tested against Enterobacterales from European hospitals analysed by geographic region and infection type (2019–2020). Eur J Clin Microbiol Infect Dis 41:477–487. https://doi.org/10.1007/s10096-022-04400-z
Kazmierczak KM, Bradford PA, Stone GG, de Jonge BLM, Sahm DF (2018) In vitro activity of ceftazidime-avibactam and aztreonam-avibactam against OXA-48-carrying Enterobacteriaceae isolated as part of the International Network for Optimal Resistance Monitoring (INFORM) Global Surveillance Program from 2012 to 2015. Antimicrob Agents Chemother 62:e00592-e1518. https://doi.org/10.1128/AAC.00592-18
Rossolini GM, Stone G, Kantecki M, Arhin FF (2022) In vitro activity of aztreonam/avibactam against isolates of Enterobacterales collected globally from ATLAS in 2019. J Glob Antimicrob Resist 30:214–221. https://doi.org/10.1016/j.jgar.2022.06.018
Wise M, Estabrook M, Arhin FF, Sahm D (2022) In vitro activities of aztreonam-avibactam and comparator agents against metallo-β-lactamase-producing enterobacterales collected during the ATLAS Global Surveillance Program, 2016–2020. 01014. Presented at: 32nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). Lisbon, Portugal
Sader HS, Castanheira M, Kimbrough JH, Kantro V, Mendes RE (2023) Aztreonam/avibactam activity against a large collection of carbapenem-resistant Enterobacterales (CRE) collected in hospitals from Europe, Asia and Latin America (2019–21). JAC Antimicrob Resist 5:dlad032. https://doi.org/10.1093/jacamr/dlad032
Newman TJ, Dreslinski GR, Tadros SS (1985) Safety profile of aztreonam in clinical trials. Rev Infect Dis 7(Suppl 4):S648-655. https://doi.org/10.1093/clinids/7.supplement_4.s648
National Institute of Diabetes and Digestive and Kidney Diseases (2017) LiverTox: clinical and research information on drug-induced liver injury. Aztreonam. https://www.ncbi.nlm.nih.gov/books/NBK548462/. Accessed 6 Dec 2022
Center for Drug Evaluation and Research (2015) Application number: 206829Orig1s000. Ceftolozane-tazobactam. Clinical Pharmacology and Biopharmaceutics Review(s), 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206829orig1s000clinpharmr.pdf. Accessed 7 Nov 2017
Bhavnani SM, Trang M, Griffith DC, Lomovskaya O, Hammel JP, Loutit JS, Cammarata SK, Dudley MN, Ambrose PG, Rubino CM (2022) Pharmacokinetic-pharmacodynamic target attainment analyses as support for meropenem-vaborbactam dosing regimens and susceptibility breakpoints. Antimicrob Agents Chemother 66:e0213021. https://doi.org/10.1128/aac.02130-21
Das S, Li J, Armstrong J, Learoyd M, Edeki T (2015) Randomized pharmacokinetic and drug-drug interaction studies of ceftazidime, avibactam, and metronidazole in healthy subjects. Pharmacol Res Perspect 3:e00172. https://doi.org/10.1002/prp2.172
Vishwanathan K, Mair S, Gupta A, Atherton J, Clarkson-Jones J, Edeki T, Das S (2014) Assessment of the mass balance recovery and metabolite profile of avibactam in humans and in vitro drug-drug interaction potential. Drug Metab Dispos 42:932–942. https://doi.org/10.1124/dmd.113.055335
Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J (2011) The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 50:99–110. https://doi.org/10.2165/11539220-000000000-00000
Toutain PL, Gandia P, Pelligand L, Ferran AA, Lees P, Bousquet-Melou A, Concordet D (2023) Biased computation of probability of target attainment for antimicrobial drugs. CPT: pharmacometrics & systems pharmacology 12:681–689. https://doi.org/10.1002/psp4.12929
Xie R, Chan PLS, McFadyen LM, Raber S (2020) Development of a simultaneous population pharmacokinetic model for aztreonam-avibactam. 990. Presented at: 30th European Congress of Clinical Microbiology and Infectious Diseases. Paris, France
Shaw E, Rombauts A, Tubau F, Padulles A, Camara J, Lozano T, Cobo-Sacristan S, Sabe N, Grau I, Rigo-Bonnin R, Dominguez MA, Carratala J (2018) Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother 73:1104–1106. https://doi.org/10.1093/jac/dkx496
Hobson CA, Bonacorsi S, Fahd M, Baruchel A, Cointe A, Poey N, Jacquier H, Doit C, Monjault A, Tenaillon O, Birgy A (2019) Successful treatment of bacteremia due to NDM-1-producing Morganella morganii with Aztreonam and ceftazidime-avibactam combination in a pediatric patient with hematologic malignancy. Antimicrob Agents Chemother 63:e02463-e12418. https://doi.org/10.1128/AAC.02463-18
Benchetrit L, Mathy V, Armand-Lefevre L, Bouadma L, Timsit JF (2020) Successful treatment of septic shock due to NDM-1-producing Klebsiella pneumoniae using ceftazidime/avibactam combined with aztreonam in solid organ transplant recipients: report of two cases. Int J Antimicrob Agents 55:105842. https://doi.org/10.1016/j.ijantimicag.2019.10.023
Paul M, Carrara E, Retamar P, Tangden T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, Pulcini C, Garnacho-Montero J, Seme K, Tumbarello M, Lindemann PC, Gandra S, Yu Y, Bassetti M, Mouton JW, Tacconelli E, Rodriguez-Bano J (2022) European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect 28:521–547. https://doi.org/10.1016/j.cmi.2021.11.025
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ (2022) Infectious Diseases Society of America 2022 Guidance on the treatment of extended-spectrum beta-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis 75:187–212. https://doi.org/10.1093/cid/ciac268
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ (2022) Infectious Diseases Society of America Guidance on the treatment of AmpC beta-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 74:2089–2114. https://doi.org/10.1093/cid/ciab1013
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ (2023) Infectious Diseases Society of America 2023 Guidance on the treatment of antimicrobial resistant gram-negative infections. Clin Infect Dis. https://doi.org/10.1093/cid/ciad428
Lodise TP, O'Donnell JN, Balevic S, Liu X, Gu K, George J, Raja S, Guptill JT, Zaharoff S, Schwager N, Fowler VG, Jr., Wall A, Wiegand K, Chambers HF, Antibacterial Resistance Leadership G (2022) Pharmacokinetics of ceftazidime-avibactam in combination with aztreonam (COMBINE) in a phase 1, open-label study of healthy adults. Antimicrob Agents Chemother:e0093622. https://doi.org/10.1128/aac.00936-22
Acknowledgements
The authors would like to thank the volunteers, patients, and investigators involved in the studies included in these analyses. The authors also thank James Li and Diansong Zhou for their contributions to the population pharmacokinetic analyses.
Funding
The population pharmacokinetic and probability of target attainment analyses were sponsored by AstraZeneca. AstraZeneca’s rights to aztreonam-avibactam were acquired by Pfizer in December 2016. Medical writing support was provided by Mark Waterlow, BSc, of Onyx, a division of Prime, Knutsford, Cheshire, UK, and funded by Pfizer. Aztreonam-avibactam is being developed by Pfizer and AbbVie (following its acquisition of Allergan), with funding from the Innovative Medicines Initiative COMBACTE-CARE group (European Union; Phase IIa and Phase III REVISIT trial) and the Biomedical Advanced Research and Development Authority (United States; Phase III REVISIT and ASSEMBLE trials). All authors take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Contributions
Study concept and design: M.M., J.G.W., S.D., T.R., T.J.C., and A.L. Data analysis and interpretation: M.M., J.G.W., S.D., A.C., L.M., R.X., A.L., and S.R. Critical review and revision of the manuscript: M.M., J.G.W., S.D., T.R., T.J.C., A.C., S.R., L.M., R.X., and A.L. Final approval of the manuscript draft to be published: all authors.
Corresponding author
Ethics declarations
Ethics approval
All clinical trials included in the analyses were conducted in accordance with the Helsinki Declaration of 1975 (as revised in 1983) and approved by local/institutional ethics committees.
Conflict of interest
Shampa Das is a former employee of and shareholder in AstraZeneca. Todd Riccobene is an employee of and shareholder in AbbVie (following its acquisition of Allergan). Timothy J. Carrothers is a former employee of AbbVie and holds shares in AbbVie. James G. Wright and Andrew Cristinacce are employees of Wright Dose Ltd., which received funding from AstraZeneca for support and assistance with the population PK analyses. Merran MacPherson is a former employee of Wright Dose Ltd. and holds shares in AstraZeneca. Alison Luckey is a former contractor for AstraZeneca and a former employee of Pfizer. Susan Raber and Rujia Xie are employees of and shareholders in Pfizer. Lynn McFadyen is a former employee of Pfizer and holds shares in Pfizer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Das, S., Riccobene, T., Carrothers, T.J. et al. Dose selection for aztreonam-avibactam, including adjustments for renal impairment, for Phase IIa and Phase III evaluation. Eur J Clin Pharmacol 80, 529–543 (2024). https://doi.org/10.1007/s00228-023-03609-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03609-x