Abstract
Introduction
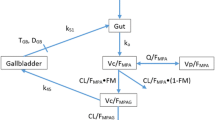
Mycophenolic acid (MPA), the active metabolite of mycophenolate mofetil (MMF), is widely used in the treatment of systemic lupus erythematosus (SLE). It has been shown that its therapeutic drug monitoring based on the area under the curve (AUC) improves treatment efficacy. MPA exhibits a complex bimodal absorption, and a double gamma distribution model has been already proposed in the past to accurately describe this phenomenon. These previous population pharmacokinetics models (POPPK) have been developed using iterative two stage Bayesian (IT2B) or non-parametric adaptive grid (NPAG) methods. However, non-linear mixed effect (NLME) approaches based on stochastic approximation expectation–maximization (SAEM) algorithms have never been published so far for this particular model. The objectives of this study were (i) to implement the double absorption gamma model in Monolix, (ii) to compare different absorption models to describe the pharmacokinetics of MMF, and (iii) to develop a limited sampling strategy (LSS) to estimate AUC in pediatric SLE patients.
Material and methods
A data splitting of full pharmacokinetic profiles sampled in 67 children extracted either from the expert system ISBA (n = 34) or the hospital Saint Louis (n = 33) was performed into train (75%) and test (25%) sets. A POPPK was developed for MPA in the train set using a NLME and the SAEM algorithm and different absorption models were implemented and compared (first order, transit, or simple and double gamma). The best limited sampling strategy was then determined in the test set using a maximum-a-posteriori Bayesian method to estimate individual PK parameters and AUC based on three blood samples compared to the reference AUC calculated using the trapezoidal rule applied on all samples and performances were assessed in the test set.
Results
Mean patient age and dose was 13 years old (5–18) and 18.1 mg/kg (7.9–47.6), respectively. MPA concentrations (764) from 107 occasions were included in the analysis. A double gamma absorption with a first-order elimination from the central compartment best fitted the data. The optimal LSS with samples at 30 min, 2 h, and 3 h post-dose exhibited good performances in the test set (mean bias − 0.32% and RMSE 21.0%).
Conclusion
The POPPK developed in this study adequately estimated the MPA AUC in pediatric patients with SLE based on three samples. The double absorption gamma model developed with the SAEM algorithm showed very accurate fit and reduced computation time.



Similar content being viewed by others
Data availability
Supporting information is available in the additional files and further supporting data is available from the authors on reasonable request.
Code availability
Mlxtran model code is available in supplementary materials.
References
Tsokos GC (2011) Systemic Lupus Erythematosus. N Engl J Med 365(22):2110–2121
Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I et al (2020) 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 79(6):713–723
Bergan S, Brunet M, Hesselink DA, Johnson-Davis KL, Kunicki PK, Lemaitre F et al (2021) Personalized therapy for mycophenolate: consensus report by the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther Drug Monit 43(2):150–200
Godron-Dubrasquet A, Woillard JB, Decramer S, Fila M, Guigonis V, Tellier S et al (2021) Mycophenolic acid area under the concentration-time curve is associated with therapeutic response in childhood-onset lupus nephritis. Pediatr Nephrol Berl Ger 36(2):341–347
Kiang TKL, Ensom MHH (2018) Population pharmacokinetics of mycophenolic acid: an update. Clin Pharmacokinet 57(5):547–558
Woillard JB, Debord J, Marquet P (2018) Comment on “Population pharmacokinetics of mycophenolic acid: an update.” Clin Pharmacokinet 57(9):1211–1213
Prémaud A, Weber LT, Tönshoff B, Armstrong VW, Oellerich M, Urien S et al (2011) Population pharmacokinetics of mycophenolic acid in pediatric renal transplant patients using parametric and nonparametric approaches. Pharmacol Res 63(3):216–224
Rong Y, Wichart J, Hamiwka L, Kiang TKL (2023) Significant effects of renal function on mycophenolic acid total clearance in pediatric kidney transplant recipients with population pharmacokinetic modeling. Clin Pharmacokinet 62(9):1289–1303
Wang XX, Liu W, Zheng T, Park JM, Smith DE, Feng MR (2017) Population pharmacokinetics of mycophenolic acid and its glucuronide metabolite in lung transplant recipients with and without cystic fibrosis. Xenobiotica Fate Foreign Compd Biol Syst 47(8):697–704
Zhao W, Fakhoury M, Deschênes G, Roussey G, Brochard K, Niaudet P et al (2010) Population pharmacokinetics and pharmacogenetics of mycophenolic acid following administration of mycophenolate mofetil in de novo pediatric renal-transplant patients. J Clin Pharmacol 50(11):1280–1291
Woillard JB, Saint-Marcoux F, Monchaud C, Youdarène R, Pouche L, Marquet P (2015) Mycophenolic mofetil optimized pharmacokinetic modelling, and exposure-effect associations in adult heart transplant recipients. Pharmacol Res 99:308–315
Prémaud A, Debord J, Rousseau A, Le Meur Y, Toupance O, Lebranchu Y et al (2005) A double absorption-phase model adequately describes mycophenolic acid plasma profiles in de novo renal transplant recipients given oral mycophenolate mofetil. Clin Pharmacokinet 44(8):837–847
Prémaud A, Le Meur Y, Debord J, Szelag JC, Rousseau A, Hoizey G et al (2005) Maximum a posteriori bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther Drug Monit 27(3):354–361
Saint-Marcoux F, Royer B, Debord J, Larosa F, Legrand F, Deconinck E et al (2009) Pharmacokinetic modelling and development of Bayesian estimators for therapeutic drug monitoring of mycophenolate mofetil in reduced-intensity haematopoietic stem cell transplantation. Clin Pharmacokinet 48(10):667–675
Zahr N, Amoura Z, Debord J, Hulot JS, Saint-Marcoux F, Marquet P et al (2008) Pharmacokinetic study of mycophenolate mofetil in patients with systemic lupus erythematosus and design of Bayesian estimator using limited sampling strategies. Clin Pharmacokinet 47(4):277–284
Saint-Marcoux F, Guigonis V, Decramer S, Gandia P, Ranchin B, Parant F et al (2011) Development of a Bayesian estimator for the therapeutic drug monitoring of mycophenolate mofetil in children with idiopathic nephrotic syndrome. Pharmacol Res 63(5):423–431
Proost JH, Eleveld DJ (2006) Performance of an iterative two-stage Bayesian technique for population pharmacokinetic analysis of rich data sets. Pharm Res 23(12):2748–2759
Abolhassani-Chimeh R, Akkerman OW, Saktiawati AMI, Punt NC, Bolhuis MS, Subronto YW et al (2022) Population pharmacokinetic modelling and limited sampling strategies for therapeutic drug monitoring of pyrazinamide in patients with tuberculosis. Antimicrob Agents Chemother 66(7):e0000322
Sheiner LB (1984) The population approach to pharmacokinetic data analysis: rationale and standard data analysis methods. Drug Metab Rev 15(1–2):153–171
Beal SL (1984) Population pharmacokinetic data and parameter estimation based on their first two statistical moments. Drug Metab Rev 15(1–2):173–193
Delyon B, Lavielle M, Moulines E (1999) Convergence of a stochastic approximation version of the EM algorithm. Ann Stat 27(1):94–128
Liu X, Wang Y (2016) Comparing the performance of FOCE and different expectation-maximization methods in handling complex population physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn 43(4):359–370
Woillard JB, Bader-Meunier B, Salomon R, Ranchin B, Decramer S, Fischbach M et al (2014) Pharmacokinetics of mycophenolate mofetil in children with lupus and clinical findings in favour of therapeutic drug monitoring. Br J Clin Pharmacol 78(4):867–876
Beaulieu Q, Zhang D, Melki I, Baudouin V, Goldwirst L, Woillard JB et al (2022) Pharmacokinetics of mycophenolic acid and external evaluation of two limited sampling strategies of drug exposure in patients with juvenile systematic lupus erythematosus. Eur J Clin Pharmacol 78(6):1003–1010
Debord J, Risco E, Harel M, Le Meur Y, Büchler M, Lachâtre G et al (2001) Application of a gamma model of absorption to oral cyclosporin. Clin Pharmacokinet 40(5):375–382
Monolix (2019) Monolix 2019 User Guide
Mizaki T, Nobata H, Banno S, Yamaguchi M, Kinashi H, Iwagaitsu S et al (2023) Population pharmacokinetics and limited sampling strategy for therapeutic drug monitoring of mycophenolate mofetil in Japanese patients with lupus nephritis. J Pharm Health Care Sci 9(1):1
Goutelle S, Woillard JB, Neely M, Yamada W, Bourguignon L (2022) Nonparametric methods in population pharmacokinetics. J Clin Pharmacol 62(2):142–157
Sherwin CMT, Sagcal-Gironella ACP, Fukuda T, Brunner HI, Vinks AA (2012) Development of population PK model with enterohepatic circulation for mycophenolic acid in patients with childhood-onset systemic lupus erythematosus. Br J Clin Pharmacol 73(5):727–740
Rong Y, Jun H, Kiang TKL (2021) Population pharmacokinetics of mycophenolic acid in paediatric patients. Br J Clin Pharmacol 87(4):1730–1757
Marquet P, Destère A, Monchaud C, Rérolle JP, Buchler M, Mazouz H et al (2021) Clinical pharmacokinetics and Bayesian estimators for the individual dose adjustment of a generic formulation of tacrolimus in adult kidney transplant recipients. Clin Pharmacokinet 60(5):611–622
Bustad A, Terziivanov D, Leary R, Port R, Schumitzky A, Jelliffe R (2006) Parametric and nonparametric population methods: their comparative performance in analysing a clinical dataset and two Monte Carlo simulation studies. Clin Pharmacokinet 45(4):365–383
Carter AA, Rosenbaum SE, Dudley MN (1995) Review of methods in population pharmacokinetics. Clin Res Regul Aff 12(1):1–21
Jelliffe R, Schumitzky A, Bustad A, Van Guilder M, Wang X, Leary R (2004) Population pharmacokinetic and pharmacodynamic modeling. In: Krishna R,. Applications of pharmacokinetic principles in drug development. Boston, MA: Springer US 373‑404
Sheiner LB, Beal SL (1980) Evaluation of methods for estimating population pharmacokinetics parameters. I. Michaelis-Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm 8(6):553‑71
Dartois C, Brendel K, Comets E, Laffont CM, Laveille C, Tranchand B et al (2007) Overview of model-building strategies in population PK/PD analyses: 2002–2004 literature survey. Br J Clin Pharmacol 64(5):603–612
Savic RM, Mentré F, Lavielle M (2011) Implementation and evaluation of the SAEM algorithm for longitudinal ordered categorical data with an illustration in pharmacokinetics–pharmacodynamics. AAPS J 13(1):44–53
Savic R, Lavielle M (2009) Performance in population models for count data, part II: a new SAEM algorithm. J Pharmacokinet Pharmacodyn 36(4):367–379
Mbogning C, Bleakley K, Lavielle M (2015) Joint modelling of longitudinal and repeated time-to-event data using nonlinear mixed-effects models and the stochastic approximation expectation–maximization algorithm. J Stat Comput Simul 85(8):1512–1528
Lavielle M, Mbogning C (2014) An improved SAEM algorithm for maximum likelihood estimation in mixtures of non linear mixed effects models. Stat Comput 24(5):693–707
Funding
Kévin Koloskoff received funding from the “association nationale recherche technologie” (ANRT) through a “convention industrielle de formation par la recherche” thesis. This research is also supported by Exactcure. Association Nationale de la Recherche et de la Technologie, 2021/1440.
Author information
Authors and Affiliations
Contributions
KK, JBW, and SB contributed to the study conception and design. Data collection was performed by EJA and JBW. Data analysis was performed by KK, LC, and JBW. The first draft of the manuscript was written by KK and JBW, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Specific approval of an ethics committee is not needed for a non-interventional study based on anonymized data of authorized collections and written parental consent is not required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koloskoff, K., Benito, S., Chambon, L. et al. Limited sampling strategy and population pharmacokinetic model of mycophenolic acid in pediatric patients with systemic lupus erythematosus: application of a double gamma absorption model with SAEM algorithm. Eur J Clin Pharmacol 80, 83–92 (2024). https://doi.org/10.1007/s00228-023-03587-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03587-0