Abstract
Purpose
Nausea is a common and unpleasant sensation for which current therapies such as serotonin (5-HT3) antagonists are often ineffective, while also conferring a risk of potential adverse events. Isopropyl alcohol (IPA) has been proposed as a treatment for nausea. We aimed to compare IPA with 5-HT3 antagonists for the treatment of nausea across all clinical settings.
Methods
MEDLINE, EMBASE, PubMed, CENTRAL and CINAHL were searched from inception to 17 July 2023 for randomised controlled trials (RCTs) comparing inhaled IPA and a 5-HT3 antagonist for treatment of nausea. Severity and duration of nausea, rescue antiemetic use, adverse events and patient satisfaction were the outcomes sought. Risk of bias (RoB) was assessed using Cochrane RoB 2. Random-effects model was used for meta-analysis. Combination of meta-analyses and narrative review was used to synthesise findings. The evidence was appraised using GRADE.
Results
From 1242 records, 4 RCTs were included with 382 participants. Participants receiving IPA had a significantly lower mean time to 50% reduction in nausea (MD − 20.06; 95% CI − 26.26, − 13.85). Nausea score reduction at 30 min was significantly greater in the IPA group (MD 21.47; 95% CI 15.47, 27.47). IPA led to significantly reduced requirement for rescue antiemetics (OR 0.60; 95% CI 0.37, 0.95; p = 0.03). IPA led to no significant difference in patient satisfaction when compared with a 5-HT3 antagonist. The overall GRADE assessment of evidence quality ranged from very low to low.
Conclusion
IPA may provide rapid, effective relief of nausea when compared with 5-HT3 antagonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Nausea is a feeling of sickness associated with the urge to vomit [1] and has a significant impact on the patient experience and healthcare worldwide [2, 3]. Nausea and its sequelae can range from mildly uncomfortable to severe [4,5,6]. There are severe clinical difficulties in treating nausea, as measures of severity are limited to subjective patient-reported scales [7]. Current treatment of nausea usually comprises antiemetic medication, most commonly antagonists of histamine (H1), dopamine (D2) or serotonin (5-HT3) receptors [8]. 5-HT3 antagonists are frequently employed as a first-line treatment [9]. Although these treatments are widely used and considered to be the most effective agents in the post-surgical setting, they are sometimes ineffective, can be associated with adverse events and can incur sizeable financial costs for healthcare [10,11,12,13,14,15]. Deleterious side effects associated with drugs of the 5-HT3 antagonist class include headaches, constipation, abdominal pain and QTc prolongation [16, 17].
The interactions and pathways that cause nausea and vomiting are complex. Nausea is the conscious awareness of the subconscious stimulation of the part of the medulla adjacent to or part of the emetic centre. This can be caused by impulses from as follows: the chemoreceptor trigger zone (CTZ) in the nucleus tractus solitarius of the area postrema located in the midbrain, the cerebral cortex or, directly, the gastrointestinal tract [18].
Aromatherapy is the inhalation of vaporised substances to alleviate symptoms. Various aromatherapies have been recommended for the treatment of nausea [19, 20]. Isopropyl alcohol (IPA), the active component of cleaning alcohol wipes commonly found in clinical settings, is one such substance [21,22,23]. It is non-invasive, inexpensive, associated with minimal adverse events [24] and readily available across healthcare settings. The exact mechanism of action of IPA aromatherapy is not well understood. It may potentially be due to its depressant effects on the central nervous system [25]. It has been suggested that inhaled IPA may regulate neuropathways involved in the emetic reflex [26]. Peak anti-nausea effect appears to be reached at around 4 min post-inhalation [19] and continued short-lasting relief may be provided by repeated inhalation of vapour [27].
The effectiveness and safety of IPA for the treatment of nausea is not widely understood, and comparison with 5-HT3 antagonists has been limited. Although the effectiveness of aromatherapy on post-operative nausea and vomiting has been the subject of previous review [28], the use of IPA has not previously been compared with 5-HT3 antagonists across clinical settings. To determine the potential for IPA to be used for nausea in the clinical setting, we performed a systematic review and meta-analysis of randomised controlled trials (RCTs) comparing IPA inhalation to 5-HT3 antagonists for the treatment of nausea.
Methods
The methods for this study—including the review question, search strategy, inclusion and exclusion criteria and risk of bias assessment—were outlined within a protocol developed prior to the conduct of the review. This protocol was prospectively registered with PROSPERO (number CRD42021259620) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) [29] reporting guidelines. Cochrane methods [30] were utilised along with a GRADE approach (proposed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group) [31].
Search strategy and selection criteria
The PICO (population, intervention, comparator group, outcome) framework was used to formulate the research question and inclusion criteria. This review included prospective RCTs that measured the effect of both inhaled isopropyl alcohol and a 5-HT3 antagonist for the treatment of nausea, vomiting or both. Studies were excluded that compared treatments for the prophylaxis of nausea. The population comprised patients of all age groups (adult and paediatric) with nausea, in any clinical setting. The intervention was IPA inhalation via any technique. No limits were placed on number of breaths, number of repetitions or time intervals between inhalations. The comparator was 5-HT3 antagonists. The outcomes were severity of nausea or vomiting and rates of adverse effects.
A search was performed in PubMed (incorporating MEDLINE), Embase, CINAHL and the Cochrane Library (CENTRAL) databases from inception to 17 July 2023. No language, date or publication limits were set. Searches were supplemented by consultation of current contents, reviews and original research relating to the treatment of nausea identified through targeted searches of Google Scholar and PubMed. These are detailed in Doc 1: Search Strategies.
Data extraction
Screening of titles and abstracts was facilitated through use of a web application (Rayyan, Qatar Computing Research Institute, Ar-Rayyan, Qatar) [32]. Two reviewers independently screened titles and abstracts and then reviewed full texts. Disagreements were resolved by consensus. Three independent reviewers extracted relevant data from the included studies. These included research design, study setting, population characteristics, intervention characteristics, comparator characteristics, timeframe for follow-up, quantitative and qualitative outcomes, source of funding, reported conflicts of interest and methodological quality information. Data were synthesised in narrative and tabular formats. The primary outcomes were severity and duration of nausea, vomiting or both, after treatment as measured by a numerical scale. Other outcomes of interest included adverse effects, requirement of additional pharmacological anti-emetic intervention (rescue therapy) and patient satisfaction as measured with a validated scale.
Data analysis
Data analyses were performed using Stata Statistical Software: Release 15.1 College Station, TX: StataCorp LP. In this meta-analysis, for the continuous outcomes, mean differences with 95% confidence intervals (CIs) were calculated, and for the dichotomous outcome: needing nausea rescue; odds ratios (ORs) and 95% CIs were calculated for each study, and then for all the studies combined. An outcome was meta-analysed if at least two of the studies reported the outcome. The I2 statistic was used to evaluate heterogeneity (with I2 > 50% indicating significant heterogeneity) as was Cochran’s Q p value (with p value < 0.05 indicating significant heterogeneity). A random-effects model was used for all meta-analyses. A statistical significance level of 5% was adopted. Funnel plots or Egger’s tests were not presented due to lack of power with less than 10 studies. Intended subgroup analyses comparing adult and child populations were unable to be performed owing to limited data.
Risk of bias assessments were independently performed by two reviewers. The included trials were critically appraised using the Cochrane risk-of-bias tool for randomized trials (RoB 2) [30, 33]. The authors assessed risk of bias under the following domains: randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. The certainty of evidence was assessed using the GRADE approach. GRADEpro GDT software was used to rate evidence and present it within a GRADE evidence profile, consisting of a certainty assessment and summary of findings table [34, 35].
Patient and public involvement
Patients and the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
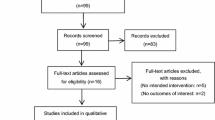
Our searches identified 1242 records, reduced to a total of 1178 after removal of duplicates. Of these, 16 were deemed relevant to the selection criteria after title and abstract screening. The subsequent full-text appraisal identified 4 studies that met the inclusion criteria. The study selection process is summarised in Fig. 1. Of the 16 retrieved full-text studies, 10 were excluded for failing to meet the inclusion criteria; either by study type, incorrect comparator, for lacking the relevant data for meta-analysis or for inability of the reviewers to find the full text. One full text could not be obtained [36]. One study was excluded due to concern for validity of study results [37]. Further details are displayed in the Characteristics of excluded studies table (Table 1) [25, 36,37,38,39,40,41,42,43,44,45,46]. Of the included four studies, each was a RCT [27, 47,48,49], and a total of 382 study participants were included in the final study cohort. Not all trials described patient demographics. Only one study reported a source of funding. Regarding the clinical setting, two of the studies were undertaken in the postoperative setting, and two in Emergency Departments (ED). The two postoperative studies included female patients who were not critically unwell (American Society of Anesthesiology class I, II or III [47, 48]), and undergoing elective laparoscopic gynaecological surgery. The postoperative studies each controlled for anaesthesia regimen. One of the included studies examined a younger cohort (maximum age limit at 65 years of age [48]) while the others included all adults. Further details can be found in the Characteristics of included studies table (Table 2).
Measures of treatment effect were mostly limited to patient-reported outcomes which were measured by scales including Verbal Numerical Rating Scales (VNRS) and Visual Analog Scales (VAS) [7, 50, 51]. Also considered was the use of additional pharmacological ‘rescue’ antiemetics. The studies used a variety of comparisons. Diversity of time points and measurement scales limited the data that could be pooled. No studies were excluded from risk of bias assessments.
Nausea duration
Two studies with 90 participants compared time to 50% nausea reduction between treatment with inhaled IPA and a 5-HT3 antagonist. The overall mean difference in time to 50% reduction in nausea score across the studies was − 20.06 min (95% CI − 26.26, − 13.85) (see Fig. 2). The overall mean time to 50% reduction in nausea score in the IPA group was 20.06 min less than that in the 5-HT3 antagonist group. Heterogeneity in the study estimates assessed using the I-squared statistic was 0% and Cochran’s Q p value was 0.691, suggesting no significant heterogeneity.
Nausea severity
The severity of nausea, as recorded on a validated scale, was reported in three of the studies. Two of these studies reported a ‘reduction in nausea’ score at 30 min following treatment. The pooled mean difference in nausea reduction at 30 min was 21.47 (95% CI 15.47, 27.47) (see Fig. 3). I-squared statistic of 0% and Cochran’s Q p value of 0.62 indicated absence of heterogeneity. April et al. [27] also found that IPA led to a 13 points lower mean nausea score (VAS) at disposition (95% CI 3, 23). Kakhki et al. [49] also examined nausea scores at 10 min. and the presence of vomiting at 10 and 30 min, and found no significant difference between groups with respect to vomiting or nausea at 10 min, but significantly more frequent vomiting in the IPA group at 30 min (38% vs 6%, p = 0.001). Winston et al. [47] reported nausea scores at specific time points post-treatment (5, 10, 15, 30, 45 and 60 min). These authors found that inhaled IPA led to significantly lower median VNRS scores of nausea at the 5-, 10- and 15-min marks when compared with a 5-HT3 antagonist (p values = 0.002, 0.015 and 0.036 respectively).
Rescue antiemetic requirement
The use of additional ‘rescue’ antiemetic therapy was compared in two of the included studies. The odds ratio of needing nausea rescue across the studies is 0.60 (95% CI 0.37, 0.95; p = 0.03) in favour of IPA, reaching the nominal statistical significance threshold. I-squared statistic was 0%, and Cochran’s Q p value was 0.57 which show absence of heterogeneity (see Fig. 4).
Adverse events
One study reported on the effect of nausea treatment with inhaled IPA or a 5-HT3 antagonist on adverse events, reporting no recorded adverse events in either group [27].
Patient satisfaction
The effect of nausea treatment method on patient satisfaction was reported by two included studies. April et al. [27] reported on overall patient satisfaction as a mean score (VAS), finding no significant difference between those exposed to IPA rather than 5-HT3 antagonist. Cotton et al. [48] measured patient satisfaction on a four-point scale and found no significant difference between the groups.
Risk of bias in studies
Assessment of risk of bias of each of the included studies was performed using the Cochrane risk-of-bias tool for randomised trials (RoB 2). The risk of bias varied across the studies, ranging from ‘some concerns’ to ‘high risk’. The RoB 2 judgements for all study results in all domains are available in the risk of bias table (Doc 3). Briefly summarised, the majority of RCTs (3/4) were considered an overall high risk of bias. The one remaining study was rated overall as ‘some concerns’ for risk of bias. A persistent area of concern was that the use of an inhaling agent precluded blinding as patients could identify which treatment they were allocated to. The Doc 2: GRADE Evidence Profile is provided in the appendix.
Discussion
This systematic review and meta-analysis of RCTs suggests that inhaled IPA may have comparable or superior effectiveness across clinical settings for the treatment of nausea when compared with 5-HT3 antagonists. Nausea treated with inhaled IPA resolved significantly faster, and nausea severity reduced when compared with those treated with a 5-HT3 antagonist. Specifically, our meta-analysis found the mean time to 50% reduction in nausea score was 20.06 min less, and the reduction in nausea score at 30 min 21.47 points greater in patients treated with inhaled IPA when compared with a 5-HT3 antagonist. Rescue antiemetic requirement was significantly lower in those treated with inhaled IPA. No significant difference was found in patient satisfaction between groups. Prior to our review, none had previously compared the use of isopropyl alcohol and 5-HT3 antagonists in the treatment of nausea in all clinical settings. However, our evidence base largely reflects the use of antiemetics in the postoperative and emergency department settings.
The findings of improved nausea duration associated with inhaled IPA when compared with 5-HT3 antagonists support the suggested utility of using this treatment method [26]. A systematic review of the effectiveness of aromatherapies for postoperative nausea and vomiting [28] found similarly that inhaled IPA appeared effective in reducing nausea duration and rescue antiemetic use when compared to ‘standard treatment’. Another systematic review comparing the effectiveness of complementary non-invasive therapies on postoperative nausea and vomiting in women undergoing laparoscopic hysterectomy [52] found that inhaled IPA when compared with a 5-HT3 antagonist was effective in altering duration of nausea; however, it did not significantly reduce the need for rescue antiemetics. The results of these reviews are mostly in agreement with our meta-analysis, in finding either equivalence between the comparators or benefit in the direction of inhaled IPA treatment.
IPA is delivered through pads soaked in the substance being placed close to the nares, through which patients inhale taking two to three deep breaths. IPA has been proven to be safe in animal models [53, 54], and no adult studies have recorded any related adverse events [22, 48]. It exhibits toxicity to human adults only with oral ingestion. Of the investigated aromatherapies, IPA has shown particularly promising results in systematic reviews [20, 52] and trials [55,56,57]. However, one trial found it to be equivalent to peppermint aromatherapy and placebo [58]. This suggests further placebo-controlled trials are required. IPA appears to be used for the treatment of nausea in various clinical settings, including EDs and post-anaesthesia care units [25, 41, 48, 59]. Delivery via inhalation is an advantage, particularly in post-operative patients who may be unable to swallow oral medications and be at a higher risk of aspiration if that occurs. Its use as a traditional treatment for nausea is said to have originated in South America [21, 22, 58].
There are limitations to this study. There was one text identified through title and abstract screening which could not be retrieved [36], and given the small evidence base, the absence of that trial may impact results. The meta-analyses had low levels of heterogeneity, so the risk of bias from this source was also low. The studies that showed changes in nausea severity in favour of the IPA group were generally at relatively brief time intervals. There was limited evidence for preferential effect of IPA at 60 min or greater.
In general, the studies included more patients who were female, relatively young and not critically unwell. These demographic factors may limit the generalisability of the results and add another source of bias. A potential source of bias arises from the fact that the majority of included studies in this review were published in the USA, thereby not accounting for potential regional differences in practices regarding care for nausea patients.
It is possible that further studies of inhaled IPA for the treatment of nausea with greater rigour in study methods could find differing results from those identified in this review. However, there was minimal inconsistency in the pooled results, improving our level of confidence in these findings. The evidence for inhaled IPA when compared with 5-HT3 antagonists in the treatment of nausea is incomplete, with no paediatric participants (age < 18) included in the review. The GRADE certainty ratings for the outcome results ranged from very low to low, with generally small sample sizes, incomplete reporting and concerning risk of bias leading to downgrading of evidence quality ratings. Inhaled IPA is inherently a difficult intervention to blind to both participants and experimenters, owing to its strong odour. Minimal inconsistency in the pooled results increases the level of confidence in the found effects. Low participant numbers in some included studies resulted in imprecision, reducing quality of evidence. However, there was little concern over publication bias. The capacity to detect bias in meta-analyses on a limited number of trials is limited [60], however, and there exists a plausibility of publication bias amongst some of these included trials which revealed perhaps surprising results. The exact method of IPA inhalation has not been yet standardised, in terms of inhaled breaths taken and intervals between inhalations, limiting comparability of the results.
Although it was not the primary focus of this analysis, the cost differences between IPA and 5-HT3 antagonists should be considered when evaluating their respective roles. Previous commentaries in Western settings have highlighted that IPA inhalation is inexpensive compared with 5-HT3 antagonists [39]. For example, ondansetron, a commonly utilised 5-HT3 antagonist costs $7.39 for four tablets [61]. While there may be variation between providers and methods of IPA delivery, this treatment method would typically cost less than $0.05 [41] for a routine hospital disinfectant swab. While this cost difference may seem small at an individual patient level, at a systems level, the cost difference may be significant. This difference may have particular significance for low-income settings.
When considering the implications of the results of this analysis, the utility of a multimodal approach to the management of nausea should be considered. Previous studies have identified that utilising a combination of drug therapies, along with non-pharmacological interventions, may be most effective in the management of PONV [62]. While this review focussed on comparative studies, the possible role of IPA in a multimodal approach that utilises interventions with a diverse array of mechanisms of actions may be an area that would benefit from further study.
As nausea is a frequent and significant issue in most clinical situations, especially in the postoperative setting, findings from this meta-analysis of RCTs provide valuable information for healthcare systems and providers. Comparative or superior treatment of nausea may be obtained through the administration of inhaled IPA relative to 5-HT3 antagonists — the current first-line treatment of nausea in many settings globally. Notably, IPA was not associated with a significant difference in patient satisfaction. Our meta-analysis suggests that IPA inhalation could be considered as a novel, effective treatment for nausea across clinical settings. However, the overall quality of the evidence included was low, and there are significant limitations to the interpretation of the findings. Future research could strengthen the literature via RCTs in paediatric populations and with larger sample sizes across multiple centres, with further data on both short- and medium-term outcomes as well as adverse effects.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CTZ:
-
Chemoreceptor trigger zone
- D2:
-
Dopamine
- ED:
-
Emergency department
- IPA:
-
Isopropyl alcohol
- GRADE:
-
Grading of Recommendations, Assessment, Development and Evaluations
- GDT:
-
Guideline development tool
- H1:
-
Histamine
- MOOSE:
-
Meta-analysis of Observational Studies in Epidemiology
- PICO:
-
Population, intervention, comparator group, outcome
- PONV:
-
Postoperative nausea and vomiting
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- QTc:
-
Corrected QT-interval
- RCT:
-
Randomised controlled trial
- RoB:
-
Risk of bias
- VAS:
-
Visual analogue scale
- VNRS:
-
Verbal numerical rating scale
- 5-HT3:
-
Serotonin
References
Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J (2012) Harrison’s Principles of Internal Medicine. McGraw-Hill, New York, NY
Gold BS, Kitz DS, Lecky JH, Neuhaus JM (1989) Unanticipated admission to the hospital following ambulatory surgery. JAMA 262(21):3008–3010
Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, Jin Z, Kovac AL, Meyer TA, Urman RD, Apfel CC, Ayad S, Beagley L, Candiotti K, Englesakis M, Hedrick TL, Kranke P, Lee S, Lipman D, Minkowitz HS, Morton J, Philip BK (2020) Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 131(2):411–448. https://doi.org/10.1213/ane.0000000000004833
Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline D (2016) Tintinalli’s emergency medicine: a comprehensive study guide. McGraw-Hill, New York, NY
Marx JA, Rosen P (2014) Rosen’s emergency medicine: concepts and clinical practice. ELsevier/Saunders, Philadelphia, PA
Miller RD, Reves JG, Glass PSA, Lubarsky DA, McEvoy MD (2005) Miller’s anaesthesia. Elsevier/Churchill Livingstone, New York, USA
Boogaerts JG, Vanacker E, Seidel L, Albert A, Bardiau FM (2000) Assessment of postoperative nausea using a visual analogue scale. Acta Anaesthesiol Scand 44(4):470–474. https://doi.org/10.1034/j.1399-6576.2000.440420.x
Longmore M, Wilkinson I, Baldwin A, Wallin E (2014) Oxford handbook of clinical medicine. Oxford University Press, London, England
Machu TK (2011) Therapeutics of 5-HT3 receptor antagonists: current uses and future directions. Pharmacol Ther 130(3):338–347. https://doi.org/10.1016/j.pharmthera.2011.02.003
Barrett TW, DiPersio DM, Jenkins CA, Jack M, McCoin NS, Storrow AB, Singleton LM, Lee P, Zhou C, Slovis CM (2011) A randomized, placebo-controlled trial of ondansetron, metoclopramide, and promethazine in adults. Am J Emerg Med 29(3):247–255. https://doi.org/10.1016/j.ajem.2009.09.028
Cieslak GD, Watcha MF, Phillips MB, Pennant JH (1996) The dose-response relation and cost-effectiveness of granisetron for the prophylaxis of pediatric postoperative emesis. Anesthesiology 85(5):1076–1085. https://doi.org/10.1097/00000542-199611000-00016
Furyk JS, Meek RA, Egerton-Warburton D (2015) Drugs for the treatment of nausea and vomiting in adults in the emergency department setting. Cochrane Database Syst Rev 2015(9):Cd010106. https://doi.org/10.1002/14651858.CD010106.pub2
Cooke CE, Mehra IV (1994) Oral ondansetron for preventing nausea and vomiting. Am J Hosp Pharm 51(6):762–771
Jokinen J, Smith AF, Roewer N, Eberhart LH, Kranke P (2012) Management of postoperative nausea and vomiting: how to deal with refractory PONV. Anesthesiol Clin 30(3):481–493. https://doi.org/10.1016/j.anclin.2012.07.003
Egerton-Warburton D, Meek R, Mee MJ, Braitberg G (2014) Antiemetic use for nausea and vomiting in adult emergency department patients: randomized controlled trial comparing ondansetron, metoclopramide, and placebo. Ann Emerg Med 64(5):526-532.e521. https://doi.org/10.1016/j.annemergmed.2014.03.017
Benedict CR, Arbogast R, Martin L, Patton L, Morrill B, Hahne W (1996) Single-blind study of the effects of intravenous dolasetron mesylate versus ondansetron on electrocardiographic parameters in normal volunteers. J Cardiovasc Pharmacol 28(1):53–59. https://doi.org/10.1097/00005344-199607000-00009
Bryson JC (1992) Clinical safety of ondansetron. Semin Oncol 19(6 Suppl 15):26–32
Hall JE, Guyton AC (2011) Guyton and Hall textbook of medical physiology. Saunders Elsevier, Philadelphia, PA
Safajou F, Soltani N, Taghizadeh M, Amouzeshi Z, Sandrous M (2020) The effect of combined inhalation aromatherapy with lemon and peppermint on nausea and vomiting of pregnancy: a double-blind, randomized clinical trial. Iran J Nurs Midwifery Res 25(5):401–406. https://doi.org/10.4103/ijnmr.IJNMR_11_19
Fellowes D, Barnes K, Wilkinson S (2004) Aromatherapy and massage for symptom relief in patients with cancer. Cochrane Database Syst Rev (2):Cd002287. https://doi.org/10.1002/14651858.CD002287.pub2
Spencer KW (2004) Isopropyl alcohol inhalation as treatment for nausea and vomiting. Plast Surg Nurs 24(4):149–154. https://doi.org/10.1097/00006527-200410000-00005
Mamaril ME, Windle PE, Burkard JF (2006) Prevention and management of postoperative nausea and vomiting: a look at complementary techniques. J Perianesth Nurs 21(6):404–410. https://doi.org/10.1016/j.jopan.2006.09.007
Tsai SS, Wang HH, Chou FH (2020) The effects of aromatherapy on postpartum women: a systematic review. J Nurs Res 28(3):e96. https://doi.org/10.1097/jnr.0000000000000331
Lua PL, Zakaria NS (2012) A brief review of current scientific evidence involving aromatherapy use for nausea and vomiting. J Altern Complement Med 18(6):534–540. https://doi.org/10.1089/acm.2010.0862
Merritt BA, Okyere CP, Jasinski DM (2002) Isopropyl alcohol inhalation: alternative treatment of postoperative nausea and vomiting. Nurs Res 51(2):125–128. https://doi.org/10.1097/00006199-200203000-00009
Wang SM, Hofstadter MB, Kain ZN (1999) An alternative method to alleviate postoperative nausea and vomiting in children. J Clin Anesth 11(3):231–234. https://doi.org/10.1016/s0952-8180(99)00035-5
April MD, Oliver JJ, Davis WT, Ong D, Simon EM, Ng PC, Hunter CJ (2018) Aromatherapy versus oral ondansetron for antiemetic therapy among adult emergency department patients: a randomized controlled trial. Ann Emerg Med 72(2):184–193. https://doi.org/10.1016/j.annemergmed.2018.01.016
Hines S, Steels E, Chang A, Gibbons K (2018) Aromatherapy for treatment of postoperative nausea and vomiting. Cochrane Database Syst Rev 3(3):Cd007598. https://doi.org/10.1002/14651858.CD007598.pub3
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160. https://doi.org/10.1136/bmj.n160
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) (2021) Cochrane handbook for systematic reviews of intervensions. In: ed. Cochrane
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926. https://doi.org/10.1136/bmj.39489.470347.AD
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5(1):210. https://doi.org/10.1186/s13643-016-0384-4
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366:l4898. https://doi.org/10.1136/bmj.l4898
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, Brignardello-Petersen R, Carrasco-Labra A, De Beer H, Hultcrantz M, Kuijpers T, Meerpohl J, Morgan R, Mustafa R, Skoetz N, Sultan S, Wiysonge C, Guyatt G, Schünemann HJ (2020) GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 119:126–135. https://doi.org/10.1016/j.jclinepi.2019.10.014
Steurer J (2018) [Not Available]. Praxis (Bern 1994) 107(14):788–789. https://doi.org/10.1024/1661-8157/a002994
Allam M (2020) Comparative study between ondansetron, isopropyl alcohol inhalation and super hydration in treatment of postoperative emesis after laparoscopic appendectomy. J Anesth Clin Res 11:941
Dalrymple RA (2020) Inhaling isopropyl alcohol from alcohol wipes was amore effective antiemetic than oral ondansetron in nauseated adults. Arch Dis Child Educ Pract Ed 105(3):190–191. https://doi.org/10.1136/archdischild-2019-316913
Lindblad AJ, Ting R, Harris K (2018) Inhaled isopropyl alcohol for nausea and vomiting in the emergency department. Can Fam Physician 64(8):580
Radford KD, Fuller TN, Bushey B, Daniel C, Pellegrini JE (2011) Prophylactic isopropyl alcohol inhalation and intravenous ondansetron versus ondansetron alone in the prevention of postoperative nausea and vomiting in high-risk patients. AANA J 79(4 Suppl):S69-74
Veldhuis P, Melse M, Mullaart N (2021) Implementation of isopropyl alcohol (IPA) inhalation as the first-line treatment for nausea in the emergency department: practical advantages and influence on the quality of care. Int J Emerg Med 14(1):15. https://doi.org/10.1186/s12245-021-00334-z
Verma DK, Bansal S, Sharma P, Sundararaman P (2018) Control of postoperative nausea and vomiting in oral and maxillofacial surgery patients with isopropyl alcohol: a prospective randomized clinical trial. J Maxillofac Oral Surg 17(4):576–581. https://doi.org/10.1007/s12663-018-1094-3
Teran L, Hawkins JK (2007) The effectiveness of inhalation isopropyl alcohol vs granisetron for the prevention of postoperative nausea and vomiting. AANA J 75(6)
Shilpa H, Shwetha Odeyar S, Shruti RR, Ramesh Kumar P, Kotre PL, Sultana S (2023) Prophylactic isopropyl alcohol inhalation and intravenous ondansetron versus ondansetron alone in prevention of post operative nausea and vomiting in patients undergoing laparoscopic surgeries-a prospective randomised comparative study. Eur J Mol Clin Med 10(01)
Van Vooren J, Semahge B (2021) For treatment of nausea in the ambulatory setting, is inhaled isopropyl alcohol as effective as ondansetron? Evidence-Based Practice 24(5):10
Nct (2022) Inhaled isopropyl alcohol for treatment of nausea. https://clinicaltrials.gov/study/NCT05418244
Winston AW, Rinehart RS, Riley GP, Vacchiano CA, Pellegrini JE (2003) Comparison of inhaled isopropyl alcohol and intravenous ondansetron for treatment of postoperative nausea. Aana j 71(2):127–132
Cotton JW, Rowell LR, Hood RR, Pellegrini JE (2007) A comparative analysis of isopropyl alcohol and ondansetron in the treatment of postoperative nausea and vomiting from the hospital setting to the home. Aana j 75(1):21–26
Rezvani Kakhki B, Ghasemi T, Vafadar Moradi E, Abbasi Shaye Z, Mousavi SM (2022) Aromatherapy with isopropyl alcohol versus intravenous ondansetron in management of mild brain trauma nausea and vomiting; a randomized clinical trial. Arch Acad Emerg Med 10(1):e87. https://doi.org/10.22037/aaem.v10i1.1792
Ho K, Spence J, Murphy MF (1996) Review of pain-measurement tools. Ann Emerg Med 27(4):427–432. https://doi.org/10.1016/s0196-0644(96)70223-8
Meek R, Egerton-Warburton D, Mee MJ, Braitberg G (2015) Measurement and monitoring of nausea severity in emergency department patients: a comparison of scales and exploration of treatment efficacy outcome measures. Acad Emerg Med 22(6):685–693. https://doi.org/10.1111/acem.12685
Hewitt V, Watts R (2009) The effectiveness of non-invasive complementary therapies in reducing postoperative nausea and vomiting following abdominal laparoscopic surgery in women: a systematic review. JBI Libr Syst Rev 7(19):850–907. https://doi.org/10.11124/01938924-200907190-00001
Gill MW, Burleigh-Flayer HD, Strother DE, Masten LW, McKee RH, Tyler TR, Gardiner TH (1995) Isopropanol: acute vapor inhalation neurotoxicity study in rats. J Appl Toxicol 15(2):77–84. https://doi.org/10.1002/jat.2550150204
Ohashi Y, Nakai Y, Ikeoka H, Koshimo H, Esaki Y, Horiguchi S, Teramoto K, Nakaseko H (1988) An experimental study on the respiratory toxicity of isopropyl alcohol. J Appl Toxicol 8(1):67–71. https://doi.org/10.1002/jat.2550080111
Beadle KL, Helbling AR, Love SL, April MD, Hunter CJ (2016) Isopropyl alcohol nasal inhalation for nausea in the emergency department: a randomized controlled trial. Ann Emerg Med 68(1):1-9.e1. https://doi.org/10.1016/j.annemergmed.2015.09.031
Pellegrini J, DeLoge J, Bennett J, Kelly J (2009) Comparison of inhalation of isopropyl alcohol vs promethazine in the treatment of postoperative nausea and vomiting (PONV) in patients identified as at high risk for developing PONV. AANA J 77(4):293–299
Langevin P, Brown M (1997) A simple, innocuous, and inexpensive treatment for postoperative nausea and vomiting. In: Anesthesia and Analgesiaed. WILLIAMS & WILKINS 351 WEST CAMDEN ST, BALTIMORE, MD 21201-2436, ppS15-S15
Anderson LA, Gross JB (2004) Aromatherapy with peppermint, isopropyl alcohol, or placebo is equally effective in relieving postoperative nausea. J Perianesth Nurs 19(1):29–35. https://doi.org/10.1016/j.jopan.2003.11.001
Hunt R, Dienemann J, Norton HJ, Hartley W, Hudgens A, Stern T, Divine G (2013) Aromatherapy as treatment for postoperative nausea: a randomized trial. Anesth Analg 117(3):597–604. https://doi.org/10.1213/ANE.0b013e31824a0b1c
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Warehouse C (2023) Ondansetron Sandoz 4mg tablets 4. In: Home/Prescriptionsed. Chemist Warehouse
Chandrakantan A, Glass PS (2011) Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth 107(Suppl 1):i27-40. https://doi.org/10.1093/bja/aer358
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Conceptualisation, All authors. Methodology, JSK, JGK, FJT, DIW. Formal analysis and investigation, JSK, JGK, JG, SW, TM, JJ, SE. Writing — original draft, JSK, JGK, SB, SE, JJ. Writing — review and editing, All authors. Funding acquisition, None. Resources, JSK, JGK, JG, JJ, SE, FT, DW. Supervision, JJ, CO, SB, JH, AG, FT, DW.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Registration and protocol
The study was prospectively registered with PROSPERO under the registration number CRD42021259620, which can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=259620.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kimber, J.S., Kovoor, J.G., Glynatsis, J.M. et al. Isopropyl alcohol inhalation versus 5-HT3 antagonists for treatment of nausea: a meta-analysis of randomised controlled trials. Eur J Clin Pharmacol 79, 1525–1535 (2023). https://doi.org/10.1007/s00228-023-03560-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03560-x