Abstract
Background and objectives
Tacrolimus (TAC) has been increasingly used in patients with non-transplant settings. Because of its large between-subject variability, several population pharmacokinetic (PPK) studies have been performed to facilitate individualized therapy. This review summarized published PPK models of TAC in non-transplant patients, aiming to clarify factors affecting PKs of TAC and identify the knowledge gap that may require further research.
Methods
The PubMed, Embase databases, and Cochrane Library, as well as related references, were searched from the time of inception of the databases to February 2023, to identify TAC population pharmacokinetic studies modeled in non-transplant patients using a non-linear mixed-effects modeling approach.
Results
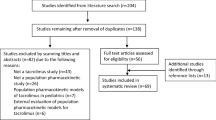
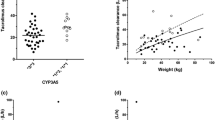
Sixteen studies, all from Asian countries (China and Korea), were included in this study. Of these studies, eleven and four were carried out in pediatric and adult patients, respectively. One-compartment models were the commonly used structural models for TAC. The apparent clearance (CL/F) of TAC ranged from 2.05 to 30.9 L·h−1 (median of 14.9 L·h−1). Coadministered medication, genetic factors, and weight were the most common covariates affecting TAC-CL/F, and variability in the apparent volume of distribution (V/F) was largely explained by weight. Coadministration with Wuzhi capsules reduced CL/F by about 19 to 43%. For patients with CYP3A5*1*1 and *1*3 genotypes, the CL/F was 39–149% higher CL/F than patients with CYP3A5*1*1.
Conclusion
The optimal TAC dosage should be adjusted based on the patient's co-administration, body weight, and genetic information (especially CYP3A5 genotype). Further studies are needed to assess the generalizability of the published models to other ethnic groups. Moreover, external validation should be frequently performed to improve the clinical practicality of the models.



Similar content being viewed by others
Data availability
The datasets supporting the results of this study are included in the article/ supplementary materials and are available upon request to the corresponding author for further inquiries.
References
Rauch MC, San Martin A, Ojeda D et al (2009) Tacrolimus causes a blockage of protein secretion which reinforces its immunosuppressive activity and also explains some of its toxic side-effects. Transpl Immunol 22(1–2):72–81
Wu B, Tong J, Ran Z (2020) Tacrolimus Therapy in Steroid-Refractory Ulcerative Colitis: A Review. Inflamm Bowel Dis 26(1):24–32
Hao GX, Song LL, Zhang DF et al (2020) Off-label use of tacrolimus in children with glomerular disease: Effectiveness, safety and pharmacokinetics. Br J Clin Pharmacol 86(2):274–284
Ong SC, Gaston RS (2021) Thirty Years of Tacrolimus in Clinical Practice. Transplantation 105(3):484–495
Sikma MA, van Maarseveen EM, van de Graaf EA et al (2015) Pharmacokinetics and Toxicity of Tacrolimus Early After Heart and Lung Transplantation. Am J Transplant 15(9):2301–2313
Yu M, Liu M, Zhang W et al (2018) Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr Drug Metab 19(6):513–522
Shuker N, van Gelder T, Hesselink DA (2015) Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando) 29(2):78–84
Lu YX, Su QH, Wu KH et al (2015) A population pharmacokinetic study of tacrolimus in healthy Chinese volunteers and liver transplant patients. Acta Pharmacol Sin 36(2):281–288
Li CJ, Li L (2015) Tacrolimus in preventing transplant rejection in Chinese patients–optimizing use. Drug Des Devel Ther 9(1):473–485
Mercè B, Teun VG, Anders Å et al (2019) Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit 41(3):261–307
Abdel Jalil M, Abdullah N, Alsous M et al (2021) Population pharmacokinetic studies of digoxin in adult patients: a systematic review. Eur J Drug Metab Pharmacokinet 46(3):325–342
Mould DR, Upton RN (2013) Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2(4):e38
Brooks E, Tett SE, Isbel NM et al (2016) Population Pharmacokinetic Modelling and Bayesian Estimation of Tacrolimus Exposure: Is this Clinically Useful for Dosage Prediction Yet? Clin Pharmacokinet 55(11):1295–1335
Campagne O, Mager DE, Tornatore KM (2019) Population Pharmacokinetics of Tacrolimus in Transplant Recipients: What Did We Learn About Sources of Interindividual Variabilities? J Clin Pharmacol 59(3):309–325
Kirubakaran R, Stocker SL, Hennig S et al (2020) Population Pharmacokinetic Models of Tacrolimus in Adult Transplant Recipients: A Systematic Review. Clin Pharmacokinet 59(11):1357–1392
Nanga TM, Doan TTP, Marquet P et al (2019) Toward a robust tool for pharmacokinetic-based personalization of treatment with tacrolimus in solid organ transplantation: A model-based meta-analysis approach. Br J Clin Pharmacol 85(12):2793–2823
Jahan A, Prabha R, Chaturvedi S et al (2015) Clinical efficacy and pharmacokinetics of tacrolimus in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 30(11):1961–1967
Lingfei H, Junyan W, Jufei Y et al (2019) Impact of CYP3A4/5 and ABCB1 polymorphisms on tacrolimus exposure and response in pediatric primary nephrotic syndrome. Pharmacogenomics 20(15):1071–1083
Wang J, Huang L, Gao P et al (2021) Diltiazem on tacrolimus exposure and dose sparing in Chinese pediatric primary nephrotic syndrome: impact of CYP3A4, CYP3A5, ABCB1, and SLCO1B3 polymorphisms. Eur J Clin Pharmacol 77(1):71–77
Zhu L, Yang J, Zhang Y et al (2015) Effects of CYP3A5 genotypes, ABCB1 C3435T and G2677T/A polymorphism on pharmacokinetics of Tacrolimus in Chinese adult liver transplant patients. Xenobiotica 45(9):840–846
Ji E, Kim MG, Oh JM (2018) CYP3A5 genotype-based model to predict tacrolimus dosage in the early postoperative period after living donor liver transplantation. Ther Clin Risk Manag 14(10):2119–2126
Antignac M, Barrou B, Farinotti R et al (2007) Population pharmacokinetics and bioavailability of tacrolimus in kidney transplant patients. Br J Clin Pharmacol 64(6):750–757
Tuteja S, Alloway RR, Johnson JA et al (2001) The effect of gut metabolism on tacrolimus bioavailability in renaltransplant recipients1,2. Transplantation 71(9):1303–1307
Kanji S, Hayes M, Ling A et al (2015) Reporting Guidelines for Clinical Pharmacokinetic Studies: The ClinPK Statement. Clin Pharmacokinet 54(7):783–795
Dartois C, Brendel K, Comets E et al (2007) Overview of model-building strategies in population PK/PD analyses: 2002–2004 literature survey. Br J Clin Pharmacol 64(5):603–612
Abdel Jalil MH, Abdullah N, Alsous MM et al (2020) A systematic review of population pharmacokinetic analyses of digoxin in the paediatric population. Br J Clin Pharmacol 86(7):1267–1280
Li Z-R, Wang C-Y, Zhu X et al (2021) Population pharmacokinetics of levetiracetam: a systematic review. Clin Pharmacokinet 60(3):305–318
Minghao L, Minglu W, Xu Z et al (2022) Tacrolimus Population Pharmacokinetic Model in Adult Chinese Patients with Nephrotic Syndrome and Dosing Regimen Identification Using Monte Carlo Simulations. Ther Drug Monit 44(5):615–624
Li L, Zhu M, Li DY et al (2021) Dose tailoring of tacrolimus based on a non-linear pharmacokinetic model in children with refractory nephrotic syndrome. Int Immunopharmacol 98(9):107827
Chen X, Wang D, Xu H et al (2020) Initial dose optimization of tacrolimus for children with systemic lupus erythematosus based on the CYP3A5 polymorphism and coadministration with Wuzhi capsule. J Clin Pharm Ther 45(2):309–317
Chen X, Wang DD, Xu H et al (2020) Optimization of initial dosing scheme of tacrolimus in pediatric refractory nephrotic syndrome patients based on CYP3A5 genotype and coadministration with wuzhi-capsule. Xenobiotica 50(5):606–613
Lu T, Zhu X, Xu S et al (2019) Dosage Optimization Based on Population Pharmacokinetic Analysis of Tacrolimus in Chinese Patients with Nephrotic Syndrome. Pharm Res 36(3):45
Chen X, Wang D, Xu H et al (2020) Population pharmacokinetics model and initial dose optimization of tacrolimus in children and adolescents with lupus nephritis based on real world data. Exp Ther Med 20(2):1423–1430
Wang D, Chen X, Xu H et al (2019) Population pharmacokinetics of tacrolimus in pediatric patients with systemic-onset juvenile idiopathic arthritis: Initial dosage recommendations. Exp Ther Med 18(6):4653–4660
Wang D, Lu J, Li Q et al (2019) Population pharmacokinetics of tacrolimus in pediatric refractory nephrotic syndrome and a summary of other pediatric disease models. Exp Ther Med 17(5):4023–4031
Huang Q, Lin X, Wang Y et al (2022) Tacrolimus pharmacokinetics in pediatric nephrotic syndrome: A combination of population pharmacokinetic modelling and machine learning approaches to improve individual prediction. Front Pharmacol 13(11):942129
Wang DD, Lu JM, Li Q et al (2018) Population pharmacokinetics of tacrolimus in paediatric systemic lupus erythematosus based on real-world study. J Clin Pharm Ther 43(4):476–483
Huang L, Liu Y, Jiao Z et al (2020) Population pharmacokinetic study of tacrolimus in pediatric patients with primary nephrotic syndrome: A comparison of linear and nonlinear Michaelis-Menten pharmacokinetic model. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 143(1):105199
Wang X, Han Y, Chen C et al (2019) Population pharmacokinetics and dosage optimization of tacrolimus in pediatric patients with nephrotic syndrome. Int J Clin Pharmacol Ther 57(3):125–134
Hao GX, Huang X, Zhang DF et al (2018) Population pharmacokinetics of tacrolimus in children with nephrotic syndrome. Br J Clin Pharmacol 84(8):1748–1756
Liu J, Guo YP, Jiao Z et al (2020) Population Pharmacokinetic Analysis of Tacrolimus in Adult Chinese Patients with Myasthenia Gravis: A Prospective Study. Eur J Drug Metab Pharmacokinet 45(4):453–466
Chen YS, Liu ZQ, Chen R et al (2017) Population pharmacokinetic analysis of tacrolimus in Chinese myasthenia gravis patients. Acta Pharmacol Sin 38(8):1195–1204
Shim MK, Kim JM, Choi KE et al (2016) Population pharmacokinetic analysis of oral tacrolimus in patients with glomerulonephritis. Int J Clin Pharmacol Ther 1(1):110–115
Zhao CY, Jiao Z, Mao JJ et al (2016) External evaluation of published population pharmacokinetic models of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol 81(5):891–907
Woillard JB, Mourad M, Neely M et al (2017) Tacrolimus Updated Guidelines through popPK Modeling: How to Benefit More from CYP3A Pre-emptive Genotyping Prior to Kidney Transplantation. Front Pharmacol 8(8):358
Zhu L, Wang H, Sun X et al (2014) The Population Pharmacokinetic Models of Tacrolimus in Chinese Adult Liver Transplantation Patients. J Pharm (Cairo) 2014(1):713650
Sam WJ, Tham LS, Holmes MJ et al (2006) Population pharmacokinetics of tacrolimus in whole blood and plasma in asian liver transplant patients. Clin Pharmacokinet 45(1):59–75
Guk J, Chae D, Park K (2017) Relationship between body weight and postmenstrual age in a Korean pediatric population. Transl Clin Pharmacol 25(2):101–105
Anderson BJ, Holford NH (2008) Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48(1):303–332
Birdwell KA, Decker B, Barbarino JM et al (2015) Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther 98(1):19–24
Bansal S (2020) Therapeutic drug monitoring of tacrolimus in kidney transplantation. Indian Journal of Transplantation 14(1):8–14
Tamashiro EY, Felipe CR, Genvigir FDV et al (2017) Influence of CYP3A4 and CYP3A5 polymorphisms on tacrolimus and sirolimus exposure in stable kidney transplant recipients. Drug Metab Pers Ther 32(2):89–95
Dong Y, Xu Q, Li R et al (2022) CYP3A7, CYP3A4, and CYP3A5 genetic polymorphisms in recipients rather than donors influence tacrolimus concentrations in the early stages after liver transplantation. Gene 809(1):146007
Naito T, Mino Y, Aoki Y et al (2015) ABCB1 genetic variant and its associated tacrolimus pharmacokinetics affect renal function in patients with rheumatoid arthritis. Clin Chim Acta 445(32):79–84
Wei H, Tao X, Di P et al (2013) Effects of traditional chinese medicine Wuzhi capsule on pharmacokinetics of tacrolimus in rats. Drug Metab Dispos 41(7):1398–1403
Qin XL, Chen X, Wang Y et al (2014) In vivo to in vitro effects of six bioactive lignans of Wuzhi tablet (Schisandra sphenanthera extract) on the CYP3A/P-glycoprotein-mediated absorption and metabolism of tacrolimus. Drug Metab Dispos 42(1):193–199
Qin XL, Chen X, Zhong GP et al (2014) Effect of Tacrolimus on the pharmacokinetics of bioactive lignans of Wuzhi tablet (Schisandra sphenanthera extract) and the potential roles of CYP3A and P-gp. Phytomedicine 21(5):766–772
Lempers VJ, Martial LC, Schreuder MF et al (2015) Drug-interactions of azole antifungals with selected immunosuppressants in transplant patients: strategies for optimal management in clinical practice. Curr Opin Pharmacol 24(1):38–44
Zahir H, McLachlan AJ, Nelson A et al (2005) Population pharmacokinetic estimation of tacrolimus apparent clearance in adult liver transplant recipients. Ther Drug Monit 27(4):422–430
Press RR, Ploeger BA, Den Hartigh J et al (2009) Explaining variability in tacrolimus pharmacokinetics to optimize early exposure in adult kidney transplant recipients. Ther Drug Monit 31(2):187–197
Wang ML, Tao YY, Sun XY et al (2021) Estrogen profile- and pharmacogenetics-based lamotrigine dosing regimen optimization: Recommendations for pregnant women with epilepsy. Pharmacol Res 169(7):105610
Acknowledgements
The authors would like to thank Wang Ming-Lu from Shengjing Hospital, China Medical University, China, for her invaluable advice and support.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81673510 and 82073936) and Outstanding Scientific Fund of Shengjing Hospital (Nos. M0779). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Cheng-bin Wang wrote the main manuscript text and Cheng-bin Wang and Yu-jia Zhang prepared figures and tables. Ming-Ming Zhao and Limei Zhao contributed to critically revising the manuscript. All authors contributed to the work, approved of the submitted version, and voiced their support for the publishing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, CB., Zhang, Yj., Zhao, MM. et al. Population pharmacokinetic analyses of tacrolimus in non-transplant patients: a systematic review. Eur J Clin Pharmacol 79, 897–913 (2023). https://doi.org/10.1007/s00228-023-03503-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03503-6