Abstract
Objective
The aim of this study was to investigate the effect of dapagliflozin (DAPA) on the rate of heart failure rehospitalization in patients with acute myocardial infarction (AMI) and type 2 diabetes mellitus (T2DM).
Methods
AMI patients with T2DM from CZ-AMI registry between January 2017 and January 2021 were enrolled in this study. Patients were stratified into DAPA users and non-DAPA users. The primary outcome was the incidence of heart failure rehospitalization. Kaplan–Meier analysis and Cox regressions were performed to evaluate the prognostic significance of DAPA. Propensity score matching (PSM) was performed to minimize the bias of confounding factors and facilitate the comparability between groups. The enrolled patients were matched with a propensity score of 1:1.
Results
A total of 961 patients were included, and 132 (13.74%) heart failure rehospitalizations occurred during a median follow-up of 540 days. In the Kaplan–Meier analysis, DAPA users had a statistically significantly lower rate of heart failure rehospitalization than non-DAPA users (p < 0.0001). Multivariate Cox analysis showed that DAPA was an independent protective factor for heart failure rehospitalization risk after discharge (HR = 0.498, 95% CI = 0.296 ~ 0.831, p = 0.001). After 1:1 propensity score matching, survival analysis showed a lower cumulative risk of heart failure rehospitalization in DAPA users than in non-DAPA users (p = 0.0007). In-hospital and continued use of DAPA remained significantly associated with a reduced risk of heart failure rehospitalization (HR = 0.417, 95% CI = 0.417 ~ 0.838, p = 0.001). Results were consistent across sensitivity and subgroup analyses.
Conclusion
In patients with diabetic AMI, in-hospital and continued use of DAPA after discharge were associated with a significant lower risk of heart failure rehospitalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF), affecting approximately 40 million people worldwide, is most likely to be caused by coronary artery atherosclerotic heart disease [1,2,3,4]. Over the past 40 years, the prevalence of HF caused by myocardial infarction (MI) has increased by 26% and 48% in men and women, respectively [5]. It is estimated that more than 8 million people aged over 18 will be affected by HF by 2030 [6, 7]. Despite effective treatments, the prognosis for patients with HF remains poor [8]. HF is the leading cause of hospitalization in adults, with a 1-year mortality rate of 10–35% in various cohorts [9,10,11,12]. In the past, neurohormonal antagonist drugs (renin-angiotensin system inhibitors, β blockers, and mineralocorticoid receptor antagonists) have laid a cornerstone for the pharmaceutic treatment of HF [13].
Sodium-glucose cotransport protein 2 (SGLT2) inhibitors, originally developed as glucose-lowering agents for the treatment of type 2 diabetes mellitus (T2DM), can decrease the risk of death and other adverse outcomes in patients with chronic HF and reduced ejection fraction (i.e., left ventricular ejection fraction ≤ 40%), or chronic kidney disease, despite the presence of T2DM. Current clinical guidelines strongly recommend the use of SGLT2 inhibitors in patients with chronic HF and reduced ejection fraction [14,15,16]. The mechanisms by which SGLT2 inhibitors improve HF outcomes are still being investigated, presumably involving regulation on hemodynamics [17, 18], myocardial energy and loading, endothelial function and inflammation, and progression of renal disease [18,19,20,21]. The EMPAREG OUTCOMES trial showed that SGLT2 inhibitors improved cardiovascular mortality in MI [22, 23]. However, the long-term outcomes of SGLT2 inhibitors in AMI patients are unclear.
The aim of this study was to investigate the relationship between dapagliflozin (DAPA), a SGLT2 inhibitor, and rehospitalization for HF after MI in patients with AMI combined with T2DM.
Materials and methods
Ethics
The study was approved by the institutional ethics committee of the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University (No. 2020-KY253-01). All patients enrolled into the current study provided signed informed consent.
Study participants
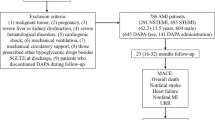
In total, 2291 AMI patients from CZ-AMI Registry (Changzhou AMI Registry, ChiCTR1800014583) between January 2017 and January 2021 were initially included in this study. Briefly, CZ-AMI Registry was a single-center, retrospective, observational cohort study of patients with AMI. The study was conducted in the Department of Cardiology, Changzhou No. 2 People’s Hospital.
Included were those who (1) had T2DM; (2) had undergone coronary angiography and percutaneous coronary intervention treatment during hospitalization; and (3) had an age of ≥ 18 years.
AMI was diagnosed according to the status of myocardial necrosis [24]. The following criteria supported the diagnosis of myocardial infarction: at least one cardiac biomarker value (preferably cardiac troponin [cTnI]) was above the 99th percentile upper reference limit (URL), and at least one of the following: (1) symptoms of ischemia; (2) new or presumed new significant ST-segment T-wave (ST-T) changes or new left bundle branch block (LBBB); (3) development of pathological Q waves in the ECG; (4) new loss of live myocardium or new imaging evidence of localized ventricular wall motion abnormalities; (5) identification of intracoronary thrombus by angiography or autopsy.
T2DM was diagnosed as follows [25]: (1) fasting blood glucose levels on another day ≥ 126 mg/dL; (2) alternatively, typical symptoms and non-fasting blood glucose ≥ 200 mg/dL; (3) a 2-h blood glucose level of 200 mg/dL in an oral glucose tolerance test (OGTT). Therefore, a total of 984 eligible individuals included in the study.
Exclusion criteria included (1) use of other kinds of SGLT2 inhibitors (5 patients used empagliflozin); (2) use of SGLT2 inhibitors before admission (n = 3); and (3) unexpected discontinuation of DAPA after discharge for various reasons (3 patients discontinued the drug because of adverse reactions, and 12 patients for other reasons). Finally, a total of 961 patients were included in the current study.
Patients were then stratified into DAPA users (DAPA group) and non-DAPA users (DAPA-Free group). DAPA users received oral DAPA 10 mg (Tablet Forxiga 10 mg, AstraZeneca, Sweden) once daily. Non-DAPA users received other kinds of glucose-lowering drugs (including sulfonylureas, glinides, α-glucosidase inhibitors, dipeptidyl-peptidase IV [DPP-4] inhibitors, insulin). Since all enrolled patients used contrast agents during the procedural, no patients used metformin. The study flowchart is illustrated in Fig. 1.
Data collections
Using an electronic medical system, we followed up all the patients for general condition, vital signs, procedural-related indicators, medication use, outpatient visits, and rehospitalization. Each patient’s weight and height were collected to calculate body mass index (BMI) by dividing weight (kg) by height squared (m). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in a calm state at admission. Prior to study procedures, the patient’s fasting blood indices were recorded, including white blood cell (WBC), neutrophil percentage, hemoglobin, blood albumin, blood uric acid, thyroid stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), hemoglobin A1c (HbA1c), and blood glucose levels. For N-terminal pro–brain natriuretic peptide (NT-pro BNP) and troponin-T (TNT), the maximal value was used during hospitalization. From the computerized case system, we gathered information about emergency percutaneous coronary intervention (e-PCI), criminal vascularization, stent placement, and intraoperative hypotension.
Endpoints
The primary endpoint event was readmission to hospital for HF. The definition of HF was as follows: (1) patients with symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality and (2) corroborated by at least one of the following: (a) plasma B-type natriuretic peptide (BNP) > 35 pg/mL or N-terminal B-type natriuretic peptide (NT-pro BNP) > 125 pg/mL; (b) evidence of cardiogenic pulmonary or systemic congestion obtained by imaging examination (such as chest radiography and echocardiography) or hemodynamic monitoring (such as right heart catheterization and pulmonary artery catheterization) [26, 27]. Secondary endpoint events were adverse drug events (including volume depletion, hyperkalemia, and ketoacidosis) and all-cause mortality.
Statistical analyses
The missing variables are shown in Supplementary Table 1. Multiple imputation was used to give each missing variable a value. First, several datasets containing all missing variables were generated. Second, these datasets were used to build several complementary models, usually generalized linear models. Third, these models were integrated together and their performances were evaluated. Finally, the complete dataset was put out [28, 29]. Categorical variables were described using frequencies and percentages, and differences between groups were identified using chi-square tests or Fisher’s exact test. Continuous variables were represented as mean ± standard deviation or mean of median and interquartile range (IQR), and compared using Student’s t-test or Mann–Whitney u-test. Kaplan–Meier analysis and Cox regressions were performed to evaluate the prognostic significance of DAPA. For survival analysis, “day 0” represented the day that the patient was admitted for AMI. Follow-up time was defined as the time from “day 0” to the occurrence of endpoints (HF rehospitalization or all-cause mortality). Propensity score matching (PSM) was performed to minimize the bias of confounding factors and facilitate the comparability between groups. Age, gender, and Killip heart functional classification were included as matching variables. A 1:1 PSM, with greedy nearest neighbor matching and caliper 0.01, was employed for matching with sex to eliminate bias and compensate for the effect of potential confounders. Standardized mean difference (SMD) was used to compare the baseline characteristics of the two groups.
Statistical analysis was carried out using R software (version 4.1.2). Graphs were created using R software and GraphPad Prism (version 8.3.0). p < 0.05 was deemed as statistically different.
Results
Baseline characteristics
From January 2017 through January 2021, 2291 patients with AMI admitted to The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University were retrospectively collected into this study initially. Among them, 961 adult AMI patients with T2DM who received interventional therapy were finally included. There were 275 (28.6%) patients in DAPA group and 686 (71.4%) patients in DAPA-Free group. Baseline clinical data between the two groups are shown and compared in Table 1. A more detailed comparison of baseline clinical data between the two groups is provided in Supplementary Table 2.
Before matching, the proportion of men was higher in the DAPA group than in the DAPA-Free group (p = 0.003), while the mean age was considerably lower in the DAPA group (p < 0.001). The proportion of Killip class ≥ 3 showed no difference between the DAPA group and the DAPA-Free group (p = 0.126) (Table 1).
Medication use
Except for the use of ACEI/ARB, there were no significant differences in other medication uses between the two groups before matching (Supplementary Table 2). We additionally analyzed the percentage of patients using ACE/ARBs, beta-blockers, and MRAs in patients with reduced EF (EF ≤ 40%). There were no significant differences between the two groups (Supplementary Table 3). Other glucose-lowering drugs used are listed in in Supplementary Table 4. There was no significant difference between the two groups.
Follow-up and primary endpoint
The median follow-up time was 540 days. The DAPA group showed a lower HF rehospitalization rate than the DAPA-Free group (6.9% vs. 16.5%; p < 0.001; Table 1). In the matched cohort, the HF rehospitalization rate was also lower significantly in the DAPA group than in the DAPA-Free group (5.6% vs. 15.2%; p = 0.001; Table 1).
Kaplan–Meier survival analysis showed a higher rate of HF rehospitalization in the DAPA-Free group than in the DAPA group (log-rank p < 0.0001, Fig. 2). In male subgroups, the rate of HF rehospitalization was higher in the DAPA-Free group than in the DAPA group, regardless of whether the ejection fraction was below 50% (log-rank p = 0.0005, 0.0020, 0.0042, Fig. 2). However, in the female subgroup, there was no significant difference in the rate of HF rehospitalization between the DAPA and DAPA-Free groups (log-rank p = 0.1181, Fig. 2).
Plot of cumulative heart failure readmission rates stratified by DAPA administration. All lines have the same meaning as the labels in (A). Unstratified pressure group before PM (A); male subgroup before PM (B); female subgroup before PM (C); LVEF greater than or equal to 50% before PM (D); LVEF less than 50% before PM (E)
Five risk-predicting models were also developed based on the results of Cox regression analysis. In model 1, the use of DAPA reduced the risk of rehospitalization for HF in AMI patients by 61.2% (HR = 0.388, 95% CI: 0.239–0.631). Model 2 was adjusted for age and sex based on model l. Model 3 was adjusted for demographic variables with p < 0.05 based on model 2. Model 4 was adjusted for laboratory variables with p < 0.05 based on model 3. Model 5 was adjusted for procedural-related variables with p < 0.05 based on model 4. Results showed that DAPA reduced the risk of HF rehospitalization in AMI patients (Table 2). Also, a univariate Cox regression analysis was performed for all statistical variables, and significant variables (p < 0.05) were included in the Cox multivariate regression analysis (Supplementary Table 5).
PSM analysis
Using a 1:1 PSM, 231 patients taking DAPA were eventually matched with 231 patients taking no DAPA. Some of the observed parameters differed between the DAPA group and the DAPA-Free group after PSM; however, the variation was less significant than that before PSM (Table 1 and Supplementary Table 2). The balance between the groups was assessed (Fig. 3). After matching, the Kaplan–Meier survival analysis showed that the DAPA-Free group had a higher HF rehospitalization rate than the DAPA group (log-rank p = 0.0007, Fig. 4). All subgroups, except for the female subgroups, demonstrated a reduction in the rate of HF rehospitalization in AMI patients with DAPA group (log-rank p = 0.2443, Fig. 4).
Balance checks of each variable after propensity score matching analysis. Standardized differences of all the variables were illustrated. UA, uric acid; HbA1c, hemoglobin A1c; FT3, free triiodothyronine; NT-pro BNP, N-terminal pro–brain natriuretic peptide; LVEF, left ventricular ejection fraction; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure
Plot of cumulative heart failure readmission rates stratified by DAPA administration; all lines have the same meaning as the labels in A. Unstratified pressure group after PM (A); male subgroup after PM (B); female subgroup after PM (C); LVEF greater than or equal to 50% after PM (D); LVEF less than 50% after PM (E)
After PSM, series Cox regression analyses were performed and 5 risk-predicting models were created to assess the relationship between DAPA use and the risk of rehospitalization for HF after AMI. In model 1, use of DAPA reduced the risk of HF rehospitalization by 65.3% (HR = 0.347, 95% CI: 0.184–0.657, p = 0.001); in model 2 adjusted for age and sex, DAPA reduced the risk of HF rehospitalization by 67.7% (HR = 0.323, 95% CI: 0.171–0.611, p < 0.001); in model 3 adjusted for general health status (BMI), DAPA reduced the risk of HF rehospitalization by 68.3% (HR = 0.318, 95% CI: 0.168–0.603, p < 0.001); in model 4 adjusted for significant laboratory indicator variables in Table 1, DAPA reduced the risk of rehospitalization for HF by 55.7% (HR = 0.443, 95% CI: 0.221–0.887, p = 0.021); in model 5 adjusted for surgical variables in Table 1, DAPA reduced the risk of rehospitalization for HF by 58.3% (HR = 0.417, 95% CI: 0.207–0.838, p < 0.001). Also, the univariate Cox regression analysis was performed for all statistical variables after PSM, and all significant variables were absorbed into the multivariate Cox regression analysis (Supplementary Table 6). DAPA significantly reduced the risk of HF rehospitalization in patients with AMI after adjustment (Table 3). At 1 year of follow-up, the DAPA group had significantly higher left ventricular ejection fraction (LVEF) values than the DAPA-free group (p = 0.0214, Supplementary Fig. 1).
Secondary endpoints
DAPA-related adverse events were also evaluated in the entire cohort. Throughout the follow-up period, ketoacidosis occurred in 1 patient and volume depletion in 2 patients (these 3 patients discontinued DAPA and were excluded from the further analysis). Hyperkalemia was not observed in all patients using DAPA. Kaplan–Meier survival analysis also showed that all-cause mortality was higher in the DAPA-Free group than in the DAPA group in both entire and matched cohorts (Log-rank p < 0.05, Supplementary Fig. 2).
Subgroup analysis
Subgroup analysis was performed by age, sex, Killip classification, hypertension, FT3, LVEF, e-PCI, and Stent. The association between DAPA and rehospitalization for HF in various subgroups was further investigated. Subgroup analysis forest plots showed that DAPA could reduce the risk of rehospitalization for HF (Fig. 5).
Discussion
In the current study, we found that in-hospital and continued use of DAPA after discharge was associated with a significant lower risk of HF rehospitalization in patients with diabetic AMI, compared with non-DAPA users. Other independent predictors of the HF rehospitalization included age, hypertension, uric acid, and Killip class. Patients with AMI and T2DM were more likely to benefit from in-hospital and continued use of DAPA. However, prospective clinical trials with a larger sample size and a longer follow-up are still required.
During the follow-up period, a total of 150 patients were hospitalized for heart failure, with a rate of 15%. It is reported in a retrospective study with a large sample of 77,363 AMI patients [30] that the heart failure rehospitalization rate was about 22% during a 5-year follow-up. The difference in this rate may be attributed to the smaller sample size in our study, as well as the geographical, dietary, climatic factors, and patients’ compliance.
We found no significant difference in the risk of HF rehospitalization between female subgroups. There are two main possible reasons. Firstly, the prevalence of AMI is higher in male than in female patients [31]. In our study, there were 66 female patients in DAPA group. The sample size is relatively small, and therefore may contribute to the lack of significant differences in the subgroup of females. Secondly, female patients have a worse prognosis post AMI [30]. During the follow-up, some female patients may have died of various reasons and could not achieve the primary endpoint. This may have also influenced the results of subgroup analysis.
In this study, we also found that most deaths occurred within 30 days post AMI and the difference in all-cause mortality was more evident in the early phase of follow-up. In another study, AMI patients undergoing percutaneous coronary intervention (PCI) and complicated with cardiogenic shock were enrolled and analyzed. Most deaths also occurred within 30 days after AMI, which is consistent with our results [32]. In the early phase post AMI, due to severe myocardial injury, cardiac dysfunction may lead to heart rupture, or papillary muscle dysfunction or rupture. These damages may end up with HF and even sudden death. In addition, various ventricular arrhythmias may appear in the early stage after heart injury, which can also result in sudden death [33]. Therefore, patients with AMI are more likely to have serious complications in the early phase after AMI. However, this study also indicated that early use of DAPA can reduce the risk of death in AMI patients more significantly.
Recurrent myocardial infarction (MI) is often followed by chronic HF, malignant arrhythmias, and cardiovascular death [34]. SGLT2 inhibitors, a novel oral hypoglycemic agent, have shown in recent clinical studies to significantly reduce the incidence of composite cardiovascular death or worsening HF events in HF patients with mildly reduced or preserved ejection fraction over a median follow-up of 2.0–2.5 years [35, 36]. However, whether SGLT2 inhibitors are effective in the early post-infarction period lacks evidence. Furthermore, considering that patients with a long history of AMI are more likely to have shared indications for SGLT2 inhibitors (e.g., T2DM or HF), it may be hasty to determine that the benefit of SGLT2 inhibitors for patients is in the context of AMI. Despite their proximate therapeutic spectra, trials in AMI populations are needed to confirm whether their treatment effects are consistent.
Existing studies suggest that in patients with T2DM and atherosclerotic cardiovascular disease, SGLT2 inhibitors reduce cardiovascular all-cause mortality and death from renal disorders, renal replacement therapy, or doubling of the serum creatinine level [37]. In contrast, this study focused on the ability of SGLT2 inhibitor in predicting rehospitalization for HF after AMI in patients combined with T2DM. One strength of this study was the introduction of propensity score matching, which allows direct comparison between the treatment and control groups, as a randomized controlled trial does [38], as well as robust error specification of the PS model [39]. In conclusion, more clinical evidence is needed in the future to confirm whether SGLT2 inhibitors should be used earlier in AMI patients.
The incidence of adverse events in this study was not high. On the one hand, this is related to the small sample size; on the other hand, our patients were all diabetic and they regularly visited a clinic for glycemic assessment and health education, which may lower the incidence of adverse events. However, the safety of SGLT2 inhibitors still requires the verification with more clinical data.
The formation of fibrous scar tissue and ventricular remodeling after AMI, along with a progressive decrease in myocardial contractility and ultimately heart failure, have been associated with death. Renal injury in the early stages of AMI has been associated with a poor prognosis in the short term and can increase long-term mortality [40]. The development of malignant arrhythmias is an important cause of early death in AMI patients [33]. Early identification of patients at a high risk of various complications is important, and an artificial intelligence model for predicting acute kidney injury risk and a new scoring system for predicting ventricular arrhythmia risk have been developed [33, 41]. In our previous studies, we have also found that a low level of free triiodothyronine is independently associated with the short-term outcomes in patients with AMI [42]. Prevention is important, but treatment is equally vital and SGLT2 inhibitors provide a new therapeutic direction for the prognosis of AMI patients. SGLT2 inhibitors may reduce the risk of acute myocardial infarction via mechanisms responsible for attenuating neurohormonal activation, cardiomyocyte necrosis, and reperfusion injury. It may also facilitate coronary blood flow and reduce ventricular load by enhancing endothelial function and vasodilation, improving myocardial energy metabolism and contractility, and other mechanisms [43,44,45,46]. With the reversal of cardiac enlargement, rhythm abnormalities, and myocardial fibrosis, HF is finally cured [47, 48]. In addition, outside the heart, SGLT2 inhibitors may also indirectly protect the cardiorenal axis by reducing intra-glomerular pressure and increasing erythropoietin production, among many other mechanisms [49, 50].
Limitations
There are several limitations in the current study. First, this study is a single-center retrospective study with a small sample size. The randomized controlled trials with larger-size samples and longer follow-up are still required in the future. Second, the patients in the DAPA-Free group may also use several kinds of glucose-lowering drugs, the effects of which were not assessed separately. Third, the current study mainly observed effects of DAPA; the efficacy and safety of other SGLT2 inhibitors, such as canagliflozin and empagliflozin, in patients with AMI should also be evaluated. Finally, patients discontinuing DAPA use were excluded from the analysis. In fact, this discontinuation may be due to the incidence of adverse events. This might have resulted in a lower observed incidence of adverse events.
Conclusions
In patients with diabetic AMI, in-hospital and continued use of DAPA after discharge from hospital were associated with a significant lower risk of HF rehospitalization.
Data availability
The datasets and materials used in the study are available from the corresponding author.
References
Baman JR, Ahmad FS (2020) Heart failure. Jama 324(10):1015
Dassanayaka S, Jones SP (2015) Recent developments in heart failure. Circ Res 117(7):e58-63
Khatibzadeh S et al (2013) Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol 168(2):1186–1194
Dokainish H et al (2017) Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health 5(7):e665–e672
Gheorghiade M et al (2006) Navigating the crossroads of coronary artery disease and heart failure. Circulation 114(11):1202–1213
Fang N, Jiang M, Fan Y (2016) Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int J Cardiol 214:279–283
Kulshreshtha A et al (2013) Life’s simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke 44(7):1909–1914
Thorvaldsen T et al (2016) Use of evidence-based therapy and survival in heart failure in Sweden 2003–2012. Eur J Heart Fail 18(5):503–511
Ambrosy AP et al (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63(12):1123–1133
Lund LH et al (2012) Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 308(20):2108–2117
Lund LH et al (2017) Association between enrolment in a heart failure quality registry and subsequent mortality-a nationwide cohort study. Eur J Heart Fail 19(9):1107–1116
Crespo-Leiro MG et al (2018) Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 20(11):1505–1535
Savarese G et al (2022) Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol 19(2):100–116
McMurray JJV et al (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381(21):1995–2008
Packer M et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383(15):1413–1424
Heerspink HJL et al (2020) Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383(15):1436–1446
Serenelli M et al (2020) Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J 41(36):3402–3418
Nassif ME et al (2021) Empagliflozin effects on pulmonary artery pressure in patients with heart failure: Results From the EMBRACE-HF Trial. Circulation 143(17):1673–1686
Santos-Gallego CG et al (2021) Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 77(3):243–255
Lee MMY et al (2021) Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation 143(6):516–525
Omar M et al (2021) Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the Empire HF Randomized Clinical Trial. JAMA Cardiol 6(7):836–840
Sharma A et al (2021) Patient phenotypes and SGLT-2 inhibition in type 2 diabetes: insights from the EMPA-REG OUTCOME Trial. JACC Heart Fail 9(8):568–577
Kaku K et al (2022) The effect of empagliflozin on the total burden of cardiovascular and hospitalization events in the Asian and non-Asian populations of the EMPA-REG OUTCOME trial of patients with type 2 diabetes and cardiovascular disease. Diabetes Obes Metab 24(4):662–674
Thygesen K et al (2012) Third universal definition of myocardial infarction. J Am Coll Cardiol 60(16):1581–1598
Vijan S (2015) In the clinic. Type 2 diabetes. Ann Intern Med 162(5):Itc1–16
McDonagh TA et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42(36):3599–3726
Bozkurt B et al (2021) Universal definition and classification of heart failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail
Austin PC et al (2021) Missing data in clinical research: a tutorial on multiple imputation. Can J Cardiol 37(9):1322–1331
Beesley LJ et al (2021) Multiple imputation with missing data indicators. Stat Methods Med Res 30(12):2685–2700
Ezekowitz JA et al (2020) Is there a sex gap in surviving an acute coronary syndrome or subsequent development of heart failure? Circulation 142(23):2231–2239
Hanratty B et al (2000) Sex differences in risk factors, treatment and mortality after acute myocardial infarction: an observational study. J Epidemiol Community Health 54(12):912–916
Miller PE et al (2022) Clinical outcomes and cost associated with an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump in patients presenting with acute myocardial infarction complicated by cardiogenic shock. JAMA Intern Med 182(9):926–933
Sun L et al (2023) A new scoring system for predicting ventricular arrhythmia risk in patients with acute myocardial infarction. Clin Interv Aging 18:283–292
Desta L et al (2015) Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail 3(3):234–42
Solomon SD et al (2022) Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER Trial. JACC Heart Fail 10(3):184–197
Anker SD et al (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385(16):1451–1461
Cannon CP et al (2020) Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 383(15):1425–1435
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46(3):399–424
Rubin DB (2004) On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf 13(12):855–857
Gao S et al (2021) Predictive value of stress hyperglycemia ratio for the occurrence of acute kidney injury in acute myocardial infarction patients with diabetes. BMC Cardiovasc Disord 21(1):157
Cai D et al (2022) Predicting acute kidney injury risk in acute myocardial infarction patients: an artificial intelligence model using medical information mart for intensive care databases. Front Cardiovasc Med 9:964894
Sun L et al (2022) Association of plasma free triiodothyronine levels with contrast-induced acute kidney injury and short-term survival in patients with acute myocardial infarction. Endocr Connect 11(7)
Lim VG et al (2019) SGLT2 inhibitor, canagliflozin, attenuates myocardial infarction in the diabetic and nondiabetic heart. JACC Basic Transl Sci 4(1):15–26
Santos-Gallego CG et al (2019) Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol 73(15):1931–1944
Griffin M et al (2020) Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 142(11):1028–1039
Shimizu W et al (2020) Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol 19(1):148
Yurista SR et al (2019) Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail 21(7):862–873
Li C et al (2019) SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 18(1):15
Cowie MR, Fisher M (2020) SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 17(12):761–772
Maruyama T et al (2019) Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther 21(12):713–720
Acknowledgements
The authors thank all local center study personnel for data collection and entry in this study.
Funding
This study was supported by grants from Changzhou High-Level Medical Talents Training Project (2022CZBJ053, 2022CZBJ054), National Natural Science Foundation of China (Grant No.82270328), Natural Science Foundation of Jiangsu Province (BK20221229), Technology Development Fund of Nanjing Medical University (NMUB2020069), Major Research Plan of Changzhou Health Commission of Jiangsu Province of China (ZD202215), China Postdoctoral Science Funding Program (2022M720544), and Changzhou Sci & Tech Program (CE20225051).
Author information
Authors and Affiliations
Contributions
LPM, DBC, YJ, QJW, and LS participated in the design of the study. LPM, DBC, BYC, TTX, ALZ, YW, QWC, QQG, and YJ performed the study. LPM, DBC, QJW, and LS analyzed the data. LPM, DBC, YJ, and LS conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mao, L., Cai, D., Chi, B. et al. Dapagliflozin reduces risk of heart failure rehospitalization in diabetic acute myocardial infarction patients: a propensity score-matched analysis. Eur J Clin Pharmacol 79, 915–926 (2023). https://doi.org/10.1007/s00228-023-03495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03495-3