Abstract
Objectives
We aimed (1) to systematically review the efficacy of transdermal nicotine patches (NP) for postoperative analgesia, (2) to establish the current quality of evidence and assist clinical decision-making on the subject, and (3) to identify methodological limitations and the need for more well-designed studies.
Materials and methods
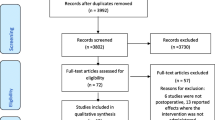
We searched six electronic databases, protocol records, and other sources without date or language restriction until March 2022. To develop the search strategy, we formulated a clinical question by using the PICOD method. Eligibility criteria included randomised placebo-controlled trials on the analgesic potential of NP for surgical procedures. This systematic review followed the PRISMA 2020 statement, and we registered the protocol in PROSPERO (#CRD42020205956).
Results
We included 10 randomised placebo-controlled trials (535 patients). The NP administered before induction of anaesthesia and at beginning of surgery reduced the pain immediately after surgery (−0.38; 95% confidence interval [CI]: −0.73 to −0.02), and 6 h (−0.34; 95% CI: −0.68 to −0.01), 12 h (−0.43; 95% CI: −0.71 to −0.15) and 24 h (−0.35; 95%CI: −0.59 to −0.10) after surgery, compared with the placebo patch (PP) group. Sensitivity testing suggests that opioid use could underestimate NP analgesia. Late demand for the first analgesic and consumption of rescue analgesics tended to be lower in the NP group.
Conclusions
The current findings suggest, with low certainty of evidence, the analgesic potential of NP for surgical procedures.
Clinical relevance
Perioperative use of NP significantly improved postoperative pain, even when opioids were administered or prescribed. Nevertheless, the clinical relevance should be interpreted with caution, owing to the effect sizes of the summary measures and methodological issues. The analgesic potential of NP as an adjuvant therapy to regulate pain and acute inflammation may offer certain clinical advantages, thus warranting further investigation.




Similar content being viewed by others
Data availability
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
Howard R, Fry B, Gunaseelan V, Lee J, Waljee J, Brummett C, Campbell D Jr, Seese E, Englesbe M, Vu J (2019) Association of Opioid Prescribing With Opioid Consumption After Surgery in Michigan. JAMA surgery 154(1):e184234. https://doi.org/10.1001/jamasurg.2018.4234
Zhao H, Yang S, Wang H, Zhang H, An Y (2019) Non-opioid analgesics as adjuvants to opioid for pain management in adult patients in the ICU: A systematic review and meta-analysis. J Crit Care 54:136–144. https://doi.org/10.1016/j.jcrc.2019.08.022
Koerber HR, Belfer I, Gold MS (2019) Nicotine evoked currents in human primary sensory neurons. J Pain 20(7):810–818. https://doi.org/10.1016/j.jpain.2019.01.005
Kalra R, Singh SP, Pena-Philippides JC, Langley RJ, Razani-Boroujerdi S, Sopori ML (2004) Immunosuppressive and anti-inflammatory effects of nicotine administered by patch in an animal model. Clin Diagn Lab Immunol 11(3):563–568. https://doi.org/10.1128/CDLI.11.3.563-568.2004
Abdrakhmanova GR, Blough BE, Nesloney C, Navarro HA, Damaj MI, Carroll FI (2010) In vitro and in vivo characterization of a novel negative allosteric modulator of neuronal nAChRs. Neuropharmacology 59(6):511–517. https://doi.org/10.1016/j.neuropharm.2010.07.006
Pathak V, Rendon IS, Lupu R, Tactuk N, Olutade T, Durham C, Stumacher R (2013) Outcome of nicotine replacement therapy in patients admitted to ICU: a randomized controlled double-blind prospective pilot study. Respir Care 58(10):1625–1629. https://doi.org/10.4187/respcare.01791
Mishriky BM, Habib AS (2014) Nicotine for postoperative analgesia: a systematic review and meta-analysis. Anesth Analg 119(2):268–275. https://doi.org/10.1213/ANE.0b013e3182a8fa7b
Ditre JW, Heckman BW, Zale EL, Kosiba JD, Maisto SA (2016) Acute analgesic effects of nicotine and tobacco in humans: a meta-analysis. Pain 157(7):1373–1381. https://doi.org/10.1097/j.pain.0000000000000572
Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, van der Valk M, Deng H, Guo W, Lehto SG, Matson D, McDermott JS, Knop J, Gaida K, Cao L, Waldon D, Albrecht BK, Boezio AA, Copeland KW, Harmange JC, Springer SK, McDonough SI (2010) Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain 149(1):33–49. https://doi.org/10.1016/j.pain.2010.01.007
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res Ed) 372:n71. https://doi.org/10.1136/bmj.n71
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clin Res Ed) 350:g7647. https://doi.org/10.1136/bmj.g7647
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174. https://doi.org/10.2307/2529310
Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Higgins J (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res Eed) 366:l4898. https://doi.org/10.1136/bmj.l4898
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
Higgins J, Thompson S, Deeks J, Altman D (2002) Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy 7(1):51–61. https://doi.org/10.1258/1355819021927674
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clin Res Ed) 343:d4002. https://doi.org/10.1136/bmj.d4002
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2022) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. Available from www.training.cochrane.org/handbook
Schünemann H, Brożek J, Guyatt G, Oxman A (2013) GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. Available from guidelinedevelopment.org/handbook
GRADEpro GDT: GRADEpro Guideline Development Tool [Software] (2020) McMaster University, (developed by Evidence Prime, Inc.). Available from gradepro.org
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin Res Ed) 336(7650):924–926
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A (2011) GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64(4):380–382. https://doi.org/10.1016/j.jclinepi.2010.09.011
Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, Brignardello-Petersen R, Carrasco-Labra A, De Beer H, Hultcrantz M, Kuijpers T, Meerpohl J, Morgan R, Mustafa R, Skoetz N, Sultan S, Wiysonge C, Guyatt G, Schünemann HJ, GRADE Working Group (2020) GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 119:126–135. https://doi.org/10.1016/j.jclinepi.2019.10.014
Habib AS, White WD, El Gasim MA, Saleh G, Polascik TJ, Moul JW, Gan TJ (2008) Transdermal nicotine for analgesia after radical retropubic prostatectomy. Anesth Analg 107(3):999–1004. https://doi.org/10.1213/ane.0b013e31816f2616
Hong D, Conell-Price J, Cheng S, Flood P (2008) Transdermal nicotine patch for postoperative pain management: a pilot dose-ranging study. Anesth Analg 107(3):1005–1010. https://doi.org/10.1213/ane.0b013e318163204f
Turan A, White PF, Koyuncu O, Karamanliodlu B, Kaya G, Apfel CC (2008) Transdermal nicotine patch failed to improve postoperative pain management. Anesth Analg 107(3):1011–1017. https://doi.org/10.1213/ane.0b013e31816ba3bb
Ali AR, Sakr SA (2009) Pre-emptive Use of Transdermal Nicotine Patch in Lumber Disc Surgery: It’s Effects on Intraoperative Anaesthetic and Analgesic Requirements, Haemodynamics and Postoperative Analgesia. Aust J Basic Appl Sci 3(2):1096–1103
Olson LC, Hong D, Conell-Price JS, Cheng S, Flood P (2009) A transdermal nicotine patch is not effective for postoperative pain management in smokers: a pilot dose-ranging study. Anesth Analg 109(6):1987–1991. https://doi.org/10.1213/ANE.0b013e3181bd1612
Nagy HIA, Elkadi HW (2014) Transdermal nicotine patch as adjunctive analgesic modality to thoracic epidural analgesia for post-thoracotomy pain. Egypt J Cardiothorac Anesth 8:75–82
Esmat IM, Kassim DY (2016) Comparative study between transdermal nicotine and melatonin patches on postoperative pain relief after laparoscopic cholecystectomy, a double-blind, placebo-controlled trial. Egypt J Anaesth 32:299–307
Malaithong W, Munjupong S (2017) Efficacy of a transdermal nicotine patch in pain relief after arthroscopic shoulder surgery: A randomized controlled trial. J Med Assoc Thai 100(8):901–906
Martins Filho ED, Vasconcelos C, Oliveira F, Pereira A, Ferraz Á (2018) Evaluation of nicotine patch in pain control of patients undergoing laparoscopic cholecystectomy. Revista do Colegio Brasileiro de Cirurgioes 45(3):e1756. https://doi.org/10.1590/0100-6991e-20181756
Landim FS, Laureano Filho JR, Nascimento J, do Egito Vasconcelos BC (2020) Effectiveness of nicotine patch for the control of pain, oedema, and trismus following third molar surgery: a randomized clinical trial. Int J Oral Maxillofac Surg 49(11):1508–1517. https://doi.org/10.1016/j.ijom.2019.08.013
Zevin S, Jacob P 3rd, Benowitz N (1997) Cotinine effects on nicotine metabolism. Clin Pharmacol Ther 61(6):649–654. https://doi.org/10.1016/S0009-9236(97)90099-0
Shiffman S, Dunbar MS, Benowitz NL (2014) A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 23(7):1264–1272. https://doi.org/10.1158/1055-9965.EPI-13-1014
Jensen AA, Frølund B, Liljefors T, Krogsgaard-Larsen P (2005) Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem 48(15):4705–4745. https://doi.org/10.1021/jm040219e
Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N (2006) Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol 146(1):116–123. https://doi.org/10.1111/j.1365-2249.2006.03169.x
Gurun MS, Parker R, Eisenach JC, Vincler M (2009) The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor. Anesth Analg 108(5):1680–1687. https://doi.org/10.1213/ane.0b013e31819dcd08
Hosur V, Leppanen S, Abutaha A, Loring RH (2009) Gene regulation of alpha4beta2 nicotinic receptors: microarray analysis of nicotine-induced receptor up-regulation and anti-inflammatory effects. J Neurochem 111(3):848–858. https://doi.org/10.1111/j.1471-4159.2009.06373.x
Nakamura M, Jang IS (2010) Presynaptic nicotinic acetylcholine receptors enhance GABAergic synaptic transmission in rat periaqueductal gray neurons. Eur J Pharmacol 640(1–3):178–184. https://doi.org/10.1016/j.ejphar.2010.04.057
Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P (2010) Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth 105(2):201–207. https://doi.org/10.1093/bja/aeq113
Hosur V, Loring RH (2011) α4β2 nicotinic receptors partially mediate anti-inflammatory effects through Janus kinase 2-signal transducer and activator of transcription 3 but not calcium or cAMP signaling. Mol Pharmacol 79(1):167–174. https://doi.org/10.1124/mol.110.066381
Nirogi R, Goura V, Abraham R, Jayarajan P (2013) α4β2* neuronal nicotinic receptor ligands (agonist, partial agonist and positive allosteric modulators) as therapeutic prospects for pain. Eur J Pharmacol 712(1–3):22–29. https://doi.org/10.1016/j.ejphar.2013.04.021
Sopori M (2002) Effects of cigarette smoke on the immune system. Nat Rev Immunol 2(5):372–377. https://doi.org/10.1038/nri803
Nishibe S, Wahl MI, Hernández-Sotomayor SM, Tonks NK, Rhee SG, Carpenter G (1990) Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science (New York, N.Y.) 250(4985):1253–1256. https://doi.org/10.1126/science.1700866
Arneric SP, Sullivan JP, Decker MW, Brioni JD, Bannon AW, Briggs CA, Donnelly-Roberts D, Radek RJ, Marsh KC, Kyncl J (1995) Potential treatment of Alzheimer disease using cholinergic channel activators (ChCAs) with cognitive enhancement, anxiolytic-like, and cytoprotective properties. Alzheimer Dis Assoc Disord 9(Suppl 2):50–61. https://doi.org/10.1097/00002093-199501002-00009
James JR, Nordberg A (1995) Genetic and environmental aspects of the role of nicotinic receptors in neurodegenerative disorders: emphasis on Alzheimer’s disease and Parkinson’s disease. Behav Genet 25(2):149–159. https://doi.org/10.1007/BF02196924
Birtwistle J, Hall K (1996) Does nicotine have beneficial effects in the treatment of certain diseases? Br J Nurs (Mark Allen Publishing) 5(19):1195–1202. https://doi.org/10.12968/bjon.1996.5.19.1195
Salomon AR, Marcinowski KJ, Friedland RP, Zagorski MG (1996) Nicotine inhibits amyloid formation by the beta-peptide. Biochemistry 35(42):13568–13578. https://doi.org/10.1021/bi9617264
Trauth JA, Seidler FJ, McCook EC, Slotkin TA (1999) Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res 851(1–2):9–19. https://doi.org/10.1016/s0006-8993(99)01994-0
Sopori ML, Kozak W, Savage SM, Geng Y, Soszynski D, Kluger MJ, Perryman EK, Snow GE (1998) Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology 23(2):189–204. https://doi.org/10.1016/s0306-4530(97)00076-0
McNicol ED, Ferguson MC, Hudcova J (2015) Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev 2015(6):CD003348. https://doi.org/10.1002/14651858.CD003348.pub3
Jamner LD, Girdler SS, Shapiro D, Jarvik ME (1998) Pain inhibition, nicotine, and gender. Exp Clin Psychopharmacol 6(1):96–106. https://doi.org/10.1037//1064-1297.6.1.96
Melnyk M, Casey RG, Black P, Koupparis AJ (2011) Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can Urol Assoc J 5(5):342–348. https://doi.org/10.5489/cuaj.11002
Schulz KF, Altman DG, Moher D, CONSORT Group (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clin Res Ed) 340:c332. https://doi.org/10.1136/bmj.c332
Acknowledgements
The authors acknowledge the financial support provided by the following Brazilian funding agencies: Coordination for the Improvement of Higher Education (CAPES), National Council for Scientific and Technological Development (CNPq) and Science and Technology Support Foundation of the State of Pernambuco (FACEPE). We would also like to thank the University of Pernambuco (UPE) and São Paulo State University (UNESP) for all their support.
Author information
Authors and Affiliations
Contributions
All authors made substantial contribution to the conception and design of the manuscript. DSB, AFMV, and BCEV performed the literature search and interpretation of the data. All authors drafted the work and revised it critically for important intellectual content. All authors agree to be accountable for all aspects of the study design and its content. All authors approved the final submitted version.
Corresponding author
Ethics declarations
Ethical approval
No ethical approval was required for this study since it was a systematic review.
Informed consent
No informed consent was required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva Barbirato, D., de Melo Vasconcelos, A.F., Dantas de Moraes, S.L. et al. Analgesic potential of transdermal nicotine patch in surgery: a systematic review and meta-analysis of randomised placebo-controlled trials. Eur J Clin Pharmacol 79, 589–607 (2023). https://doi.org/10.1007/s00228-023-03475-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03475-7