Abstract
Purpose
To estimate the risk of mortality and length of stay in hospitalised patients who have experienced suspected adverse drug reactions (ADRs) as compared to patients who did not experience suspected ADRs.
Methods
A systematic literature search was conducted on databases for observational and randomised controlled studies conducted in any inpatient setting that reported deaths and/or length of hospital stay in patients who had suspected ADRs and did not have suspected ADRs during hospitalisation. PRISMA guidelines were strictly followed during the review. The methodological quality of included studies was assessed using a tool designed by Smyth et al. for the studies of adverse drug reactions. The meta-analytic summary of all-cause mortality was estimated using odds ratio—OR (95% CI) and length of stay using mean difference—MD (95% CI). Both outcomes were pooled using a random effect model (DerSimonian and Laird method). Subgroup and meta-regression were performed based on study variables: study design, age group, study ward, study region, types of suspected ADRs (ADRAd—suspected ADRs that lead to hospitalisation and ADRIn—suspected ADRs that occur following hospitalisation), study duration, sample size and study period. The statistical analysis was conducted through the ‘Review manager software version 5.4.1 and JASP (Version 0.14.1)’.
Results
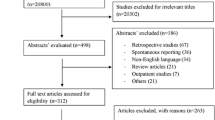
After screening 475 relevant articles, 55 studies were included in this meta-analysis. Patients having suspected ADRs had reported significantly higher odds of all-cause mortality [OR: 1.50 (95% CI: 1.21–1.86; I2 = 100%) than those patients who did not have suspected ADRs during hospitalisation. Study wards, types of suspected ADRs and sample size were observed as significant predictors of all-cause mortality (p < 0.05). Patients having suspected ADRs had reported significantly higher mean difference in hospital stay [MD: 3.98 (95% CI: 2.91, 5.05; I2 = 99%) than those patients who did not have suspected ADRs during hospitalisation. Types of suspected ADRs and study periods were observed as significant predictors of length of stay (p < 0.05).
Conclusion
Suspected ADRs significantly increase the risk of mortality and length of stay in hospitalised patients.
Systematic review registration.
CRD42020176320.



Similar content being viewed by others
Data availability
The study data are included in the main text/supplementary data file; further inquiries can be directed to the corresponding author.
References
European Medicines Agency (2017) Guideline on good pharmacovigilance practices (GVP). Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-annex-i-definitions-rev-4_en.pdf. Last assessed on 20 Nov 2022
Baldo P, Francescon S, Fornasier G (2018) Pharmacovigilance workflow in Europe and Italy and pharmacovigilance terminology. Int J Clin Pharm 40(4):748–753
Angamo MT, Chalmers L, Curtain CM, Bereznicki LR (2016) Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf 39(9):847–857
Laatikainen O, Miettunen J, Sneck S, Lehtiniemi H, Tenhunen O, Turpeinen M (2017) The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur J Clin Pharmacol 73(12):1539–1549
Miguel A, Azevedo LF, Araújo M, Pereira AC (2012) Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 21(11):1139–1354
Oscanoa TJ, Lizaraso F, Carvajal A (2017) Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis Eur J Clin Pharmacol 73(6):759–770
Patel TK, Patel PB (2016) Incidence of adverse drug reactions in Indian hospitals: a systematic review of prospectives. Curr Drug Saf 11(2):128–136
Lazarou J, Pomeranz BH, Corey PN (1998) Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279(15):1200–1205
Patel TK, Patel PB (2018) Mortality among patients due to adverse drug reactions that lead to hospitalization: a meta-analysis. Eur J Clin Pharmacol 74(6):819–832
Patel PB, Patel TK (2019) Mortality among patients due to adverse drug reactions that occur following hospitalisation: a meta-analysis. Eur J Clin Pharmacol 75(9):1293–1307
Patel TK, Patel PB, Bhalla HL, Kishore S (2022) Drug-related deaths among inpatients: a meta-analysis. Eur J Clin Pharmacol 78(2):267–278
Baek H, Cho M, Kim S, Hwang H, Song M, Yoo S (2018) Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS ONE 13(4):e0195901
Lingsma HF, Bottle A, Middleton S, Kievit J, Steyerberg EW, Marang-van de Mheen PJ (2018) Evaluation of hospital outcomes: the relation between length-of-stay, readmission, and mortality in a large international administrative database. BMC Health Serv Res 18(1):116
Beijer HJ, de Blaey CJ (2002) Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 24(2):46–54
Smyth RM, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R et al (2012) Adverse drug reactions in children–a systematic review. PLoS ONE 7(3):e24061
Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ et al. (2010) Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. 2010 Oct 25. In: Methods guide for effectiveness and comparative effectiveness reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK49407/
Panagioti M, Khan K, Keers RN, Abuzour A, Phipps D, Kontopantelis E et al (2019) Prevalence, severity, and nature of preventable patient harm across medical care settings: systematic review and meta-analysis. BMJ 366:l4185
Alexopoulou A, Dourakis SP, Mantzoukis D, Pitsariotis T, Kandyli A, Deutsch M et al (2008) Adverse drug reactions as a cause of hospital admissions: a 6-month experience in a single center in Greece. Eur J Intern Med 19(7):505–510
Amann C, Hasford J, Stausberg J (2012) Stationäre Aufnahmen wegen unerwünschter Arzneimittelereignisse (UAE): Analyse der DRG-Statistik 2006 [Hospital admission due to adverse drug events (ADE): an analysis of German routine hospital data of 2006]. Gesundheitswesen 74(10):639–644
Angamo MT, Chalmers L, Curtain CM, Yilma D, Bereznicki L (2018) Mortality from adverse drug reaction-related hospitalizations in south-west Ethiopia: a cross-sectional study. J Clin Pharm Ther 43(6):790–798
Bond CA, Raehl CL (2006) Adverse drug reactions in United States hospitals. Pharmacotherapy 26(5):601–608
Brvar M, Fokter N, Bunc M, Mozina M (2009) The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol 9:8
Camargo AL, Cardoso Ferreira MB, Heineck I (2006) Adverse drug reactions: a cohort study in internal medicine units at a university hospital. Eur J Clin Pharmacol 62(2):143–149
Chan M, Nicklason F, Vial JH (2001) Adverse drug events as a cause of hospital admission in the elderly. Intern Med J 31(4):199–205
Claret PG, Bobbia X, Renia R, Stowell A, Crampagne J, Flechet J et al (2016) Prescription errors by emergency physicians for inpatients are associated with emergency department length of stay. Therapie S0040–5957(16):30053–30061
Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP (1997) Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 277(4):301–306
Damen NL, Baines R, Wagner C, Langelaan M (2017) Medication-related adverse events during hospitalization: a retrospective patient record review study in The Netherlands. Pharmacoepidemiol Drug Saf 26(1):32–39
Darchy B, Le Mière E, Figuérédo B, Bavoux E, Domart Y (1999) Iatrogenic diseases as a reason for admission to the intensive care unit: incidence, causes, and consequences. Arch Intern Med 159(1):71–78
Davies EC, Green CF, Mottram DR, Pirmohamed M (2006) Adverse drug reactions in hospital in-patients: a pilot study. J Clin Pharm Ther 31(4):335–341
Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M (2009) Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS ONE 4(2):e4439
de Boer M, Boeker EB, Ramrattan MA, Kiewiet JJ, Dijkgraaf MG, Boermeester MA et al (2013) Adverse drug events in surgical patients: an observational multicentre study. Int J Clin Pharm 35(5):744–752
de Las SR, Díaz-Agudelo D, Burgos-Flórez FJ, Vaca C, Serrano-Meriño DV (2016) Adverse drug reactions in hospitalized Colombian children. Colomb Med (Cali) 47(3):142–147
Dequito AB, Mol PG, van Doormaal JE, Zaal RJ, van den Bemt PM, Haaijer-Ruskamp FM et al (2011) Preventable and non-preventable adverse drug events in hospitalized patients: a prospective chart review in the Netherlands. Drug Saf 34(11):1089–1100
Dormann H, Neubert A, Criegee-Rieck M, Egger T, Radespiel-Tröger M, Azaz-Livshits T et al (2004) Readmissions and adverse drug reactions in internal medicine: the economic impact. J Intern Med 255(6):653–663
Esteban Jiménez Ó, Navarro Pemán C, González Rubio F, Lanuza Giménez FJ, Montesa Lou C (2017) Análisis de la incidencia y de las características clínicas de las reacciones adversas a medicamentos de uso humano en el medio hospitalario [A study of incidence and clinical characteristics of adverse drug reactions in hospitalized patients.]. Rev Esp Salud Publica 91:e201712050
Fattinger K, Roos M, Vergères P, Holenstein C, Kind B, Masche U et al (2000) Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 49(2):158–167
Giordani F, Rozenfeld S, Martins M (2014) Adverse drug events identified by triggers at a teaching hospital in Brazil. BMC Pharmacol Toxicol 15:71
Grenouillet-Delacre M, Verdoux H, Moore N, Haramburu F, Miremont-Salamé G, Etienne G et al (2007) Life-threatening adverse drug reactions at admission to medical intensive care: a prospective study in a teaching hospital. Intensive Care Med 33(12):2150–2157
Haffner S, von Laue N, Wirth S, Thürmann PA (2005) Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf 28(5):453–464
Haukland EC, Mevik K, von Plessen C, Nieder C, Vonen B (2019) Contribution of adverse events to death of hospitalised patients. BMJ Open Qual 8(1):e000377
Hofer-Dueckelmann C, Prinz E, Beindl W, Szymanski J, Fellhofer G, Pichler M, Schuler J (2011) Adverse drug reactions (ADRs) associated with hospital admissions - elderly female patients are at highest risk. Int J Clin Pharmacol Ther 49(10):577–586
Hu Q, Qin Z, Zhan M, Chen Z, Wu B, Xu T (2020) Validating the Chinese geriatric trigger tool and analyzing adverse drug event associated risk factors in elderly Chinese patients: a retrospective review. PLoS ONE 15(4):e0232095
Ji HH, Song L, Xiao JW, Guo YX, Wei P, Tang TT et al (2018) Adverse drug events in Chinese pediatric inpatients and associated risk factors: a retrospective review using the Global Trigger Tool. Sci Rep 8(1):2573
Jolivot PA, Pichereau C, Hindlet P, Hejblum G, Bigé N, Maury E et al (2016) An observational study of adult admissions to a medical ICU due to adverse drug events. Ann Intensive Care 6(1):9
Kojima T, Matsui T, Suzuki Y, Takeya Y, Tomita N, Kozaki K et al (2020) Risk factors for adverse drug reactions in older inpatients of geriatric wards at admission: multicenter study. Geriatr Gerontol Int 20(2):144–149
Liao PJ, Mao CT, Chen TL, Deng ST, Hsu KH (2019) Factors associated with adverse drug reaction occurrence and prognosis, and their economic impacts in older inpatients in Taiwan: a nested case-control study. BMJ Open 9(5):e026771
Mehta U, Durrheim DN, Blockman M, Kredo T, Gounden R, Barnes KI (2008) Adverse drug reactions in adult medical inpatients in a South African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol 65(3):396–406
Miguel A, Marques B, Freitas A, Lopes F, Azevedo L, Pereira AC (2013) Detection of adverse drug reactions using hospital databases-a nationwide study in Portugal. Pharmacoepidemiol Drug Saf 22(8):907–913
Mjörndal T, Boman MD, Hägg S, Bäckström M, Wiholm BE, Wahlin A et al (2002) Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf 11(1):65–72
Moore N, Lecointre D, Noblet C, Mabille M (1998) Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol 45(3):301–308
Mouton JP, Njuguna C, Kramer N, Stewart A, Mehta U, Blockman M et al (2016) Adverse drug reactions causing admission to medical wards: a cross-sectional survey at 4 hospitals in South Africa. Medicine (Baltimore) 95(19):e3437
Mouton JP, Fortuin-de Smidt MC, Jobanputra N, Mehta U, Stewart A, de Waal R et al (2020) Serious adverse drug reactions at two children’s hospitals in South Africa. BMC Pediatr 20(1):3
Nazer LH, Eljaber R, Rimawi D, Hawari FI (2013) Adverse drug events resulting in admission to the intensive care unit in oncology patients: incidence, characteristics and associated cost. J Oncol Pharm Pract 19(4):298–304
Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M (2009) Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging 26(6):475–482
Park S, In Y, Suh GY, Sohn K, Kim E (2013) Evaluation of adverse drug reactions in medical intensive care units. Eur J Clin Pharmacol 69(1):119–131
Passarelli MC, Jacob-Filho W, Figueras A (2005) Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging 22(9):767–777
Pedrós C, Quintana B, Rebolledo M, Porta N, Vallano A, Arnau JM (2014) Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur J Clin Pharmacol 70(3):361–367
Pedrós C, Formiga F, Corbella X, Arnau JM (2016) Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol 72(2):219–226
Phillips AL, Nigro O, Macolino KA, Scarborough KC, Doecke CJ, Angley MT et al (2014) Hospital admissions caused by adverse drug events: an Australian prospective study. Aust Health Rev 38(1):51–57
Riaz M, Brown JD (2019) Association of adverse drug events with hospitalization outcomes and costs in older adults in the USA using the nationwide readmissions database. Pharmaceut Med 33(4):321–329
Rozenfeld S, Giordani F, Coelho S (2013) Eventos adversos a medicamentos em hospital terciário: estudo piloto com rastreadores [Adverse drug events in hospital: pilot study with trigger tool]. Rev Saude Publica 47(6):1102–1111
Rydberg DM, Holm L, Engqvist I, Fryckstedt J, Lindh JD, Stiller CO et al (2016) Adverse drug reactions in a tertiary care emergency medicine ward - prevalence, preventability and reporting. PLoS ONE 11(9):e0162948
Sánchez Muñoz-Torrero JF, Barquilla P, Velasco R, Fernández Capitan Mdel C, Pacheco N, Vicente L et al (2010) Adverse drug reactions in internal medicine units and associated risk factors. Eur J Clin Pharmacol 66(12):1257–1264
Suh DC, Woodall BS, Shin SK, Hermes-De Santis ER (2000) Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother 34(12):1373–1379
Takahashi Y, Sakuma M, Murayama H, Morimoto T (2018) Effect of baseline renal and hepatic function on the incidence of adverse drug events: the Japan Adverse Drug Events study. Drug Metab Pers Ther 33(4):165–173
Tangiisuran B, Davies JG, Wright JE, Rajkumar C (2012) Adverse drug reactions in a population of hospitalized very elderly patients. Drugs Aging 29(8):669–679
Toscano Guzmán MD, Banqueri MG, Otero MJ, Fidalgo SS, Noguera IF, Guerrero MCP (2021) Validating a trigger tool for detecting adverse drug events in elderly patients with multimorbidity (TRIGGER-CHRON). J Patient Saf 17(8):e976–e982
Trifirò G, Calogero G, Ippolito FM, Cosentino M, Giuliani R, Conforti A et al (2005) Adverse drug events in emergency department population: a prospective Italian study. Pharmacoepidemiol Drug Saf 14(5):333–340
van der Hooft CS, Sturkenboom MC, van Grootheest K, Kingma HJ, Stricker BH (2006) Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf 29(2):161–168
Vargas E, Simón J, Martin JC, Puerro M, Gonzalez-Callejo MA, Jaime M et al (1998) Effect of adverse drug reactions on length of stay in intensive care units. Clin Drug Investig 15(4):353–360
Vitorino M, Aguiar P, Sousa P (2020) In-hospital adverse drug events: analysis of trend in Portuguese public hospitals. Cad Saude Publica 36(3):e00056519
Zed PJ, Abu-Laban RB, Balen RM, Loewen PS, Hohl CM, Brubacher JR et al (2008) Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ 178(12):1563–1569
Hakkarainen KM, Hedna K, Petzold M, Hägg S (2012) Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions–a meta-analysis. PLoS ONE 7(3):e33236
Patel NS, Patel TK, Patel PB, Naik VN, Tripathi CB (2017) Hospitalizations due to preventable adverse reactions-a systematic review. Eur J Clin Pharmacol 73(4):385–398
Martins AC, Giordani F, Rozenfeld S (2014) Adverse drug events among adult inpatients: a meta-analysis of observational studies. J Clin Pharm Ther 39(6):609–620
Hackshaw A (2008) Small studies: strengths and limitations. Eur Respir J 32(5):1141–1143
Case LD, Ambrosius WT (2007) Power and sample size. Methods Mol Biol 404:377–408
Barnett AG, Page K, Campbell M, Martin E, Rashleigh-Rolls R, Halton K et al (2013) The increased risks of death and extra lengths of hospital and ICU stay from hospital-acquired bloodstream infections: a case-control study. BMJ Open 3(10):e003587
Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand SL et al (2010) Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA 303(21):2141–2147
Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J, et al. (2010) Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev (3):CD006632
Gray SL, Hart LA, Perera S, Semla TP, Schmader KE, Hanlon JT (2018) Meta-analysis of interventions to reduce adverse drug reactions in older adults. J Am Geriatr Soc 66(2):282–288
O’Mahony D (2016) Pharmacists and prevention of inappropriate prescribing in hospital. Age Ageing 45(2):181–183
Rieckert A, Teichmann AL, Drewelow E, Kriechmayr C, Piccoliori G, Woodham A et al (2019) Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA-eDS project): a survey of general practitioners’ experiences. J Am Med Inform Assoc 26(11):1323–1332
Prgomet M, Li L, Niazkhani Z, Georgiou A, Westbrook JI (2017) Impact of commercial computerized provider order entry (CPOE) and clinical decision support systems (CDSSs) on medication errors, length of stay, and mortality in intensive care units: a systematic review and meta-analysis. J Am Med Inform Assoc 24(2):413–422
Author information
Authors and Affiliations
Contributions
TKP contributed to all aspects of this manuscript, including conception and design; acquisition, analysis, interpretation of data and drafting of the manuscript. PBP contributed to the design, acquisition, analysis and interpretation of data. HLB contributed to the analysis, interpretation of data and drafting of the manuscript. PD, VB and SK contributed to the interpretation of data and drafting of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, T.K., Patel, P.B., Bhalla, H.L. et al. Impact of suspected adverse drug reactions on mortality and length of hospital stay in the hospitalised patients: a meta-analysis. Eur J Clin Pharmacol 79, 99–116 (2023). https://doi.org/10.1007/s00228-022-03419-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03419-7