Abstract
Purpose
Janus kinase (JAK) inhibitors have been developed to treat moderate to severe atopic dermatitis, but there is little evidence comparing the safety profile of these drugs. The aim of this study is to compare the relative safety of the different systemic JAK inhibitors in atopic dermatitis.
Methods
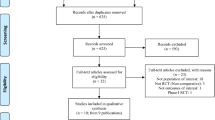
Medline, EMBASE, and clinicaltrials.gov were searched to identify phase 2/3, clinical trials (RCTs) designed to evaluate the efficacy and safety of systemic JAK inhibitors in atopic dermatitis. Outcomes were the risk of any adverse event (AE), serious AEs, AEs leading to treatment discontinuation, any infection, serious infections, herpes zoster infection, and any cardiac or vascular event.
Results
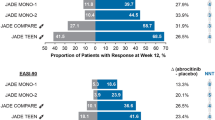
Eighteen RCTs were included. Compared with placebo, baricitinib (odds ratio [OR] 1.25, 95% credible interval [CrI] 1.03–1.55), abrocitinib (OR 1.54, 95% CrI 1.25–1.90), and upadacitinib (OR 1.46, 95% CrI 1.19–1.81) increase the risk of any adverse event. Abrocitinib (OR 1.62, 95% CrI 1.7–2.72), upadacitinib (OR 1.67, 95% CrI 1.19–2.43), and dupilumab (OR 1.69, 95% CrI 1.02–2.79) increase the risk of infections when compared with placebo. Dupilumab has a reduced risk of herpes zoster infection when compared with upadacitinib (OR 0.23; 95% CrI 0.08–0.81) No further statistically significant risk differences between treatments were identified.
Conclusions
The results suggest systemic JAK inhibitors for atopic dermatitis have a similar safety profile. However, as current data present limitations, postmarketing safety evidence will be crucial to draw definitive conclusions regarding the safety of JAK inhibitors.


Similar content being viewed by others
Availability of data and materials
Studies used in this systematic review and meta-analysis were retrieved from databases that are publicly available.
References
Wollenberg A, Barbarot S, Bieber T et al (2019) Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I [published correction appears in J Eur Acad Dermatol Venereol. 33(7):1436]. J Eur Acad Dermatol Venereol 32(5):657–682
Nemeth V, Evans J (2021) Eczema. https://www.ncbi.nlm.nih.gov/books/NBK538209/. Accessed 6 July 2022
Eichenfield LF, Tom WL, Berger TG et al (2014) Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 71(1):116–132
Guttman-Yassky E, Teixeira HD, Simpson EL et al (2021) Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials [published correction appears in Lancet. et al 2021 Jun 5;397(10290):2150] Lancet 397(10290):2151–2168
Blauvelt A, Silverberg JI, Lynde CW et al (2022) Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol 86(1):104–112
Simpson EL, Lacour JP, Spelman L et al (2020) Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol 183(2):242–255
European Medicines Agency. Human medicine European public assessment report (EPAR): Cibinqo. Direct healthcare professional communications (DHPC). https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo. Accessed 6 July 2022
European Medicines Agency. Human medicine European public assessment report (EPAR): Olumiant. Direct healthcare professional communications (DHPC). https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant. Accessed 6 July 2022
Bechman K, Subesinghe S, Norton S et al (2019) A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatol (United Kingdom) 58(10):1755–1766
European Medicines Agency. Human medicine European public assessment report (EPAR): Xeljanz. Direct healthcare professional communications (DHPC). https://www.ema.europa.eu/en/medicines/dhpc/xeljanz-tofacitinib-increased-risk-major-adverse-cardiovascular-events-malignancies-use-tofacitinib. Accessed 6 July 2022
US Food and Drug Administration. Advisory Committee Meeting: Arthritis Advisory Committee Meeting. NDA 207924 Baricitinib Janus Kinase (JAK) inhibitor for RA. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/207924Orig1s000MedR.pdf. Accessed 6 July 2022
US Food and Drug Administration. Center for Drug Evaluation and Research Application. Number 207924Orig1s000. Summary Review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/207924Orig1s000ClinPharmR.pdf. Accessed 6 July 2022
Drucker AM, Morra DE, Prieto-Merino D et al (2022) Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol 158(5):523–532
Li C, Sun X, Zhao K et al (2021) Efficacy and safety of Janus kinase inhibitors for the treatment of atopic dermatitis: a systematic review and meta-analysis published online ahead of print, 2021 Aug 27. Dermatology 1–11
Le M, Berman-Rosa M, Ghazawi FM et al (2021) Systematic review on the efficacy and safety of oral Janus kinase inhibitors for the treatment of atopic dermatitis. Front Med (Lausanne) 8:682547
University of York, Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed 6 July 2022
Hutton B, Salanti G, Caldwell DM et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
Hanifin JM, Rajka G (1980) Diagnostic features of atopic dermatitis. Acta Derm Venereol 60(92):44–47
Eichenfield LF, Tom WL, Chamlin SL et al (2014) Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 70(2): 338–51
Saeki H, Nakahara T, Tanaka A et al (2016) Clinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol 43(10):1117–1145
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:1–8
Lunn DJ, Thomas A, Best N et al (2000) WinBUGS - a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10(4):325–337
The Core Model (2018) In: Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ, editors. Network meta-analysis for decision-making. Hoboken, NJ: John Wiley & Sons, Ltd, pp 19–57
Model Fit, Model comparison and outlier detection (2018) In: Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ, editors. Network meta-analysis for decision-making. Hoboken, NJ: John Wiley & Sons, Ltd, pp 59–91
Venerito V, Lopalco G, Cacciapaglia F, Fornaro M, Iannone F (2019) A Bayesian mixed treatment comparison of efficacy of biologics and small molecules in early rheumatoid arthritis. Clin Rheumatol 38(5):1309–1317
Yan M, Kumachev A, Siu LL, Chan KK (2015) Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis. Eur J Cancer 51(12):1570–1579
Dias S, Welton NJ, Sutton AJ et al (2022) NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London: National Institute for Health and Care Excellence (NICE). https://www.ncbi.nlm.nih.gov/books/NBK310366/. Accessed 6 July 2022
Adverse events and other sparse outcome data (2018) In: Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ, editors. Network meta-analysis for decision-making. Hoboken, NJ: John Wiley & Sons, Ltd. 179–187
Bao L, Zhang H, Chan LS (2013) The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT 2(3):e24137
Wood H, Chandler A, Nezamololama N, Papp K, Gooderham MJ (2021) Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis published online ahead of print, 2021 Aug 22. Int J Dermatol. https://doi.org/10.1111/ijd.15853
Sher J, Hahn K, Paul M et al (2013) Incidence and severity of pediatric allergic/immunologic adverse drug reactions in a tertiary care center. J Allergy Clin Immunol 131(2):AB175
Chovatiya R, Paller AS (2021) JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol 148(4):927–940
Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T (2020) Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study [published correction appears in. 2021 Oct 85(4):1069 J Am Acad Dermatol 82:(4)823–831
Papp K, Szepietowski JC, Kircik L et al (2021) Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol 85(4):863–872
Silverwood RJ, Forbes HJ, Abuabara K et al (2018) Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ 361:k1786
Ascott A, Mulick A, Yu AM et al (2019) Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol 143(5):1821–1829
Nishida Y, Kubota Y, Iso H, Tamakoshi A; JACC Study Group (2019) Self-reported eczema in relation with mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study. J Atheroscler Thromb 26(9):775–782
Wang V, Boguniewicz J, Boguniewicz M, Ong PY (2021) The infectious complications of atopic dermatitis. Ann Allergy Asthma Immunol 126(1):3–12
Wang V, Keefer M, Ong PY (2019) Antibiotic choice and methicillin-resistant Staphylococcus aureus rate in children hospitalized for atopic dermatitis. Ann Allergy Asthma Immunol 122(3):314–317
Langan SM, Abuabara K, Henrickson SE, Hoffstad O, Margolis DJ (2017) Increased risk of cutaneous and systemic infections in atopic dermatitis-a cohort study. J Invest Dermatol 137(6):1375–1377
Crowson CS, Liao KP, Davis JM 3rd et al (2013) Rheumatoid arthritis and cardiovascular disease. Am Heart J 166(4):622-628.e1
Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D (2012) Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 71(9):1524–1529
Lindhardsen J, Ahlehoff O, Gislason GH et al (2011) The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 70(6):929–934
Provan SA, Lillegraven S, Sexton J et al (2020) Trends in all-cause and cardiovascular mortality in patients with incident rheumatoid arthritis: a 20-year follow-up matched case-cohort study. Rheumatology (Oxford) 59(3):505–512
Genovese M, Smolen J, Takeuchi T et al (2020) Safety profile of baricitinib for the treatment of rheumatoid arthritis over a median of 3 years of treatment: an updated integrated safety analysis. Lancet Rheumatol 2(6):e347–e357
Author information
Authors and Affiliations
Contributions
Carlos Alves: conceptualization, writing, review, and editing of the original draft. Ana Penedones: bibliographic search, review, and editing. Diogo Mendes, Francisco Batel Marques: review and editing of the original draft.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alves, C., Penedones, A., Mendes, D. et al. The safety of systemic Janus kinase inhibitors in atopic dermatitis: a systematic review and network meta-analysis. Eur J Clin Pharmacol 78, 1923–1933 (2022). https://doi.org/10.1007/s00228-022-03400-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03400-4