Abstract
Background
Urticaria following the COVID-19 vaccine was rarely reported and had a short self-limited resolution. However, there has been relatively little literature published on CSU induced by COVID-19 vaccines.
Purpose
We describe a case series of patients who experienced CSU after SARS-CoV-2 vaccination.
Methods
A retrospective case series of 10 patients referred to the Department of Clinical Pharmacology of the University of Monastir (January 2021–January 2022) and included for evaluation of urticaria after COVID-19 vaccination.
Results
The median age was 31 years and patients were mostly female. Atopy was presented in 3 patients and urticaria was accompanied by angioedema in 6 patients. The median time interval between vaccination and the onset of urticaria was 28.5 h. The offended dose was the first one in 8 patients. The resolution of the eruption was observed at least 2 months later, despite the regular use of a full dose of antihistamine in nine patients. Polynuclear leucocytosis was identified in 5 patients. Anti-TPOAb was positive in one patient after receiving the BNT162b2 vaccine. Total serum IgE was elevated in 4 patients. Skin tests for the suspected vaccine as well as the vaccine excipient were negative.
Conclusion
We add to the medical literature ten new cases of chronic spontaneous urticarial reactions following COVID-19 vaccines uncontrolled with high-dose first-generation H1 antihistamines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since March 2020, the SARS-CoV-2 pandemic has caused and affected the death of millions of people all over the world, and vaccines are considered the most effective strategy to end the pandemic [1]. Fortunately, the SARS-CoV-2 vaccines seem to be one of the most effective public health interventions to struggle against COVID-19 infection and to end the pandemic by achieving herd immunity [2]. Most reported hypersensitivity reactions after the SARS-CoV-2 vaccine include commonly self-limited localized adverse events and, rarely, systemic reactions [3]. Generally, vaccines are uncommon causes of urticaria as described by previous case reports [4,5,6,7,8]. In this context, involved vaccines were hepatitis B immunization [8], a cellular diphtheria-tetanus-pertussis (DTP) vaccines [4], Haemophilus influenzae b [5], and vaccines containing gelatin as the stabilizer such as measles, mumps, and rubella (MMR), varicella-zoster virus, and Japanese encephalitis vaccines [6, 7]. Similar to other vaccines, COVID-19 vaccines have been implicated in the onset of skin reactions such as angioedema, maculopapular eruption, and acute urticaria [9]. Most previous reports described an acute urticaria post-COVID-19 vaccination; however, chronic spontaneous urticaria (CSU) subtype has been scarcely reported with mRNA COVID-19 vaccines (Moderna, Pfizer-BioNTech BNT162b2) [10, 11] and AstraZeneca/Oxford (ChAdOx1) [12, 13]. The objective of this study is to describe a case series of patients who exhibited a CSU after SARS-CoV-2 vaccination.
Patients methods
We included all cases of urticaria post-vaccine anti-COVID-19 intake; notified to the Pharmacovigilance Unit of the Clinical Pharmacology Department of the University Hospital of Monastir (Tunisia) between March 2020 and January 2022.
The diagnostic of urticaria was established based on the proposed definition of the EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria [14]. According to these experts, the diagnosis of urticaria was based on the development of wheals (hives), angioedema, or both.
The reaction was defined as acute spontaneous urticaria (ASU) and chronic spontaneous urticaria (CSU) if the duration is less than 6 weeks and more than 6 weeks, respectively.
The causative relationship between the cutaneous reaction and the vaccine administration was established according to the probability scale of adverse drug reactions published by Naranjo et al. [15]. Demographics, medical and allergic history, reaction details, vaccine, and the outcome after subsequent vaccination were collected for each patient. An allergological workup was performed after the patient’s consent. In absence of a standardized methodology for vaccine testing, we referred to the EAACI recommendations for vaccine hypersensitivity exploration [16]. Indeed, the left-over solution in the suspected vaccine vial was used to perform skin testing. The same dilution for each vaccine type was performed on three healthcare volunteers.
Skin testing should start with an undiluted skin Prick test (SPT). If negative, intradermal testing (IDT) (0.02 mL) should follow (1:100 dilution, 1:10 dilution) [16].
In addition, we performed skin tests on the excipient (polyethylene glycol: PEG; or Polysorbate 80: PS 80) containing the suspected COVID-19 vaccine [17].
Results
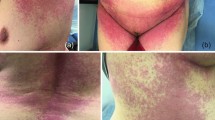
Ten patients (7 F/3 M) were included in this study. The median age was 31 years (IQR: 14–74 years). Underlying atopy was presented in three patients of whom one reported a history of penicillin hypersensitivity, the second patient had underlying asthma and food allergy, and the third was a child with cholinergic urticaria and asthma. However, none of them had a medical history of chronic spontaneous urticaria. The suspected COVID-19 vaccine was as follows: the mRNA vaccines (BNT162b2 (N = 4) and Moderna® (N = 2)), viral vector vaccine (Oxford/AstraZeneca®) (N = 2), and inactivated virus vaccine (Sinovac, CoronaVac) (N = 2). On physical examination, patients have experienced erythematous and edematous wheals, which are typically intensely pruritic and induce a burning sensation (Figs. 1 and 2). Urticaria was accompanied by angioedema (Fig. 3) in six patients: two patients following Oxford/AstraZeneca® vaccine, three patients after mRNA vaccines (BNT162b2 (N = 2) and Moderna® (N = 1)), and in the sixth case, urticaria was associated with angioedema and dyspnea following the CoronaVac administration. Among the included patients, 9 exhibited a new-onset skin reaction; however, it was a reactivation of preexisting urticaria in one of them.
The median time interval between vaccination and the onset of urticaria was 28.5 h (0.5 h to 7 days). The offended dose was the first one in eight patients and the second in two patients. All patients required antihistamines and four of them required intravenous betamethasone. The resolution of the cutaneous eruption was observed at least 2 months later (median 16 weeks: minimum 2 months–maximum 6 months), despite the regular use of antihistamine treatment (cetirizine or desloratadine) in nine patients. We recommend initially the use of a standard dose of 2nd generation H1-antihistamines as the first-line treatment for urticaria and to increase gradually, every week, at up to quadruple recommended dose, if symptoms are intolerable. Only one patient with a medical history of benign prostate hypertrophy has interrupted second-generation H1 antihistamine (levocetirizine then desloratadine) because of acute urinary retention. Laboratory tests showed polynuclear leucocytosis in five patients. Serologic markers of autoimmune disease were performed in all patients revealing positive anti-thyroperoxidase antibodies (TPOAb) in one patient 3 months after receiving the first dose of BNT162b2 (BioNTech/Pfizer). Inflammatory biomarkers (erythrocyte sedimentation rate, and C-reactive protein serum) were negative in all patients. Total serum immunoglobulin E (IgE) was elevated in four patients: three patients following BNT162b2 (BioNTech/Pfizer) (first dose: N = 2; second dose N = 1) and one patient after the first dose of CoronaVac administration.
Five patients agreed to carry out an allergological workup. Skin tests for the suspected COVID-19 vaccine as well as the vaccine excipient (PEG or PS 80) were performed and were negative in all of them. Only one patient received her next booster dose of Moderna® COVID-19 vaccine, with a relapse of urticaria associated with angioedema.
Discussion
We describe in the present study an original case series of patients who developed chronic spontaneous urticaria (CSU) thought to be related to the COVID-19 vaccine. Indeed, a clear temporal relationship was observed between the vaccine administration and the symptoms’ onset, and the absence of other attributable aetiologies. Based on the Naranjo algorithm, it is probable that the CSU was due to the COVID-19 vaccine [15]. Urticaria more commonly known as hives or wheals is a skin disorder characterized by recurrent urticaria of unknown origin that can occur with or without associated angioedema [18]. It is a frequent disorder that affects between 15 and 25% of the population [19]. When triggers (physical stimuli, stress, etc.) are identified, the urticaria is categorized as inducible, and, the urticaria is called spontaneous, otherwise. Besides, and based on the EAACI guidelines [14], urticaria is classified as acute or chronic if the duration is less than 6 weeks or more than 6 weeks, respectively. Exanthems are the most cutaneous manifestation drug-induced, followed by urticaria. This later accounted for 5.9% of all drug rashes [20]. However, drugs account for a minority of cases of chronic urticaria (9%) [21]. Drug-induced urticaria is commonly caused by nonsteroidal anti-inflammatory drugs where this later trigger can induce or aggravate preexisting CSU [22]. Regarding vaccines, the risk of hypersensitivity reaction post-vaccination is estimated to be 1.31 per million vaccine doses [3]. Cutaneous reactions mostly described are localized to the site of vaccine administration; however, a generalized reaction such as delayed urticaria, angioedema, and nonspecific skin rashes can occur in 5 to 13% of patients receiving vaccines [23]. The onset of the reaction is generally less than 4 h post-vaccination, but this delay has been reported to be up to 7 days in some cases [24]. Although rare, CSU may be associated with vaccines involving mostly the influenza vaccine (28.6%) followed by HBV vaccine (21.4%), HPV (21.4%), yellow fever (14.3%), and DTP vaccines (14.3%) [25]. Regarding anti-SARS-CoV-2 vaccines, most reported adverse events following immunization (AEFIs) are not serious [26]. The most common side effects were injection site reactions, affecting 30–70%. The generalized cutaneous reaction has been in small case series including exanthemas, vascular lesions, eczematous dermatitis, autoimmune bullous reactions, and exacerbation of chronic immune-mediated cutaneous dermatoses.
To date, anti-SARS-CoV-2 vaccine–induced CSU has been described in limited single-case reports [10,11,12,13]. Alflen et al. [11] were the first to report two cases of a relapse of CSU induced by the Moderna COVID-19 vaccine. The delay was 16 h and 30 min, respectively, after the second and the first vaccine dose. In another case, Thomas et al. [10] have described a clinical observation of a 20-year-old male, with a history of allergic reactions to sulfa drugs, who developed CSU 1 week after receiving the second dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Brooks et al. [12] have described the fourth case of CSU occurring in a 60-year-old male with a history of asthma and environmental allergies who exhibited urticarial rash 5 weeks following the first dose of AstraZeneca/Oxford COVID-19 vaccine. Recently, Suan and Lee [13] reported another case of CSU developed in a 39-year-old male with no history of atopic disorders, who developed widespread urticaria associated with swelling of hands, 2 weeks following the second AstraZeneca/Oxford COVID-19 vaccine.
Interestingly, all of the previously described observations of CSU induced by the COVID-19 vaccine were resistant to pharmacology therapy particularly high doses of H1 blocking antihistamine, similarly to the present case series.
In our study, the mRNA COVID-19 vaccine (Pfizer-BioNTech, Moderna® (mRNA-1273)) was the most suspected vaccine, followed respectively by a viral vector (vaccine Oxford/AstraZeneca®) and inactivated virus COVID-19 vaccine (Sinovac, CoronaVac).
The most implicated dose in our series was the first one (8/10), as the majority of included patients had received only the first dose (7/10). However, the re-exposure to a further dose could be associated with a recurrence of urticaria, as observed in one of the included patients. In this context, Bellinato et al. [27] showed that the frequency of urticaria was 0.3% and 0.6%, respectively after the first and the second dose of the mRNA vaccination. Moreover, these authors demonstrated that the recurrence of urticaria occurred in 3.3% of those who experienced the first reaction [27].
According to the classification of Bellinato et al. [27], the majority of our patients (9/10) exhibited new-onset skin reactions; however, it was a flare of preexisting urticarial in only one patient. The pathogenesis of CSU is complex and still incompletely understood. Several theories have been proposed; the most suggested theory is that this entity results from the activation of mast cells and basophils, which gives rise to the release of proinflammatory mediators that support the generation of urticaria [28]. However, several pieces of evidence point toward a potential autoimmune etiology in up to 50% of patients with this condition [28]. Particularly, in the case of CSU, an autoimmune pathway could be hypothesized. Indeed, the immune response induced by COVID-19 infection or vaccination could target host molecules that share structural similarities with viral epitopes. Indeed, it has been shown a similarity between COVID-19 proteins and human tissue antigens. This cross-reactivity in patients with preexisting self-tolerance deregulations may lead to the development of autoimmune disease [28].
According to Maurer et al. [29], the occurrence of CSU is rather explained by an autoimmune etiology because of its association with other autoimmune diseases (Hashimoto’s thyroiditis, vitiligo, type I diabetes) and the increased incidence of autoantibody production (positive speckled-pattern antinuclear antibodies, IgG antithyroid antigens, and IgE anti-thyroperoxidase). However, until today, absolute evidence on the mechanism of CSU is still lacking [29].
In the present series, the COVID-19 vaccine seems to be the trigger as none of the patients had a history of autoimmune diseases. It has been argued that urticaria post-vaccination can occur because of either the active vaccine component or one of the other components [3]. The anti-SARS-CoV-2 vaccines contain excipients known to be associated with the potential risk of sensitization. Indeed, the Pfizer-BioNTech vaccine contains polyethylene glycol-2000, Moderna vaccine polyethylene glycol-2000, and tromethamine and AstraZeneca vaccine including polysorbate 80. Taking account of this consideration, we performed, before receiving anti-SARS-CoV-2 vaccination, a complete medical history, and allergological workup to the suspected excipient. These explorations were negative in all tested patients, confirming the fact that urticaria is induced by the active component rather than the excipient. Up to date, the predictive value of skin tests in the case of COVID-19 vaccine–induced hypersensitivity is still unknown. Indeed, a positive immediate reaction (SPT, IDT) could be useful to confirm the diagnosis of immediate hypersensitivity reaction to a vaccine; however, a positive delayed reaction is explained by cellular immune protection. In our study, skin tests with the suspected vaccine were negative in all patients. This result is consistent with the finding of Bianchi, who found a negative immediate reading skin testing in all patients performed for whom experienced CSU and angioedema following SARS-CoV-2 vaccination. In another study, Pitlick et al. [30] found that only 12/129 patients (9.3%) with an urticaria post-vaccination had a positive skin test. Among a total of 129 patients who have performed skin testing, 101 patients have received a COVID-19 vaccine of whom 90 patients have tolerated the vaccine and the remaining 11 patients experienced minor rash after vaccination. Therefore, the study reveals the low positivity rate of COVID-19 vaccine excipient skin testing and the high rate of subsequent COVID-19 vaccine tolerance suggests a low utility of this method in the evaluation of COVID-19 vaccine hypersensitivity reactions [30]. We think that future studies are needed to better clarify the utility of SPT and IDT to investigate COVID-19 vaccine allergy. Anyway, strict avoidance of subsequent COVID-19 vaccination should be discouraged. Regarding the usefulness of premedication with antihistamine and/or corticosteroids, there is no evidence that premedication reduces the risk of subsequent CSU reactions. According to the Consensus Statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working, pretreatment with second-generation antihistamines before the second dose of COVID-19 vaccination may be considered in patients with mild allergic symptoms, such as urticaria [14].
In conclusion, through this series, we add to the medical literature ten new cases of chronic spontaneous urticarial reactions uncontrolled with high-dose first-generation H1 antihistamines. Thus, clinicians should be aware of the possibility of CSU following COVID-19 vaccines. However, the largest studies are mandatory to better understand the pathogenesis of this side effect and to update, accordingly, future recommendations in cases of CSU-induced SARS-CoV-2 vaccination.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Iannone P, Castellini G, Coclite D, Napoletano A, Fauci AJ, Iacorossi L et al (2020) The need of health policy perspective to protect healthcare workers during COVID-19 pandemic. A GRADE rapid review on the N95 respirators effectiveness. PloS One 15:e0234025
Velikova T, Georgiev T (2021) SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int 1–10
McNeil MM, DeStefano F (2018) Vaccine-associated hypersensitivity. J Allergy Clin Immunol 141:463–472
Sakaguchi M, Nakayama T, Inouye S (1998) Cases of systemic immediate-type urticaria associated with acellular diphtheria-tetanus-pertussis vaccination. Vaccine 16:1138–1140
Humphreys F (1994) Acute urticaria and angio-oedema following Haemophilus influenzae b vaccination. Br J Dermatol 131:582–583
Pool V, Braun MM, Kelso JM, Mootrey G, Chen RT, Yunginger JW et al (2002) Prevalence of anti-gelatin IgE antibodies in people with anaphylaxis after measles-mumps rubella vaccine in the United States. Pediatrics 110:e71
Sakaguchi M, Inouye S (1998) Two patterns of systemic immediate-type reactions to Japanese encephalitis vaccines. Vaccine 16:68–69
Barbaud A, Tréchot P, Reichert-Pénétrat S, Weber M, Schmutz JL (1998) Allergic mechanisms and urticaria/angioedema after hepatitis B immunization. Br J Dermatol 139:925–926
Banerji A, Wickner PG, Saff R, Stone CA, Robinson LB, Long AA et al (2021) mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract 9:1423–1437
Thomas J, Thomas G, Chatim A, Shukla P, Mardiney M (2021) Chronic spontaneous urticaria after COVID-19 vaccine. Cureus 13:e18102
Alflen C, Birch K, Shilian R, Wu SS, Hostoffer R (2021) Two cases of well controlled chronic spontaneous urticaria triggered by the Moderna COVID-19 vaccine. Allergy Rhinol Provid RI 12:21526567211026270
Brooks SG, De Jong AM, Abbaslou M, Sussman G (2022) Chronic spontaneous urticaria triggered by the AstraZeneca/Oxford COVID-19 vaccine with achieved remission: a case report. Allergy Rhinol Provid RI 13:21526567211068456
Suan D, Lee AYS (2022) Chronic spontaneous urticaria following ChAdOx1-S COVID-19 vaccination. Allergo J Int 1–2
Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM et al (2009) EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria. Allergy 64:1427–1443
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA et al (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245
Nilsson L, Brockow K, Alm J, Cardona V, Caubet JC, Gomes E et al (2017) Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol 28:628–640
Barbaud A, Garvey LH, Arcolaci A, Brockow K, Mori F, Mayorga C et al (2022) Allergies and COVID-19 vaccines: An ENDA/EAACI Position paper. Allergy
Hon KL, Leung AKC, Ng WGG, Loo SK (2019) Chronic urticaria: an overview of treatment and recent patents. Recent Pat Inflamm Allergy Drug Discov 13:27–37
Poonawalla T, Kelly B (2009) Urticaria : a review. Am J Clin Dermatol 10:9–21
Hunziker T, Künzi UP, Braunschweig S, Zehnder D, Hoigné R (1997) Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy 52:388–393
Kozel MM, Mekkes JR, Bossuyt PM, Bos JD (1998) The effectiveness of a history-based diagnostic approach in chronic urticaria and angioedema. Arch Dermatol 134:1575–1580
Shipley D, Ormerod AD (2001) Drug-induced urticaria. Recognition and treatment. Am J Clin Dermatol 2:151–158
Caubet JC, Ponvert C (2014) Vaccine allergy. Immunol Allergy Clin North Am 34(597–613):ix
Johnston MS, Galan A, Watsky KL, Little AJ (2021) Delayed localized hypersensitivity reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol 157:716–720
Magen E (2018) Chronic spontaneous urticaria following vaccination. Int J Adv Res 6:1434–1439
McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A et al (2021) Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol 85:46–55
Bellinato F, Maurelli M, Gisondi P, Girolomoni G (2021) Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med 10:5344
Bracken SJ, Abraham S, MacLeod AS (2019) Autoimmune theories of chronic spontaneous urticaria. Front Immunol 10:627
Maurer M, Khan DA, Elieh Ali Komi D, Kaplan AP (2021) Biologics for the use in chronic spontaneous urticaria: when and which. J Allergy Clin Immunol Pract 9(3):1067–1078
Pitlick MM, Sitek AN, D’Netto ME, Dages KN, Chiarella SE, Gonzalez-Estrada A et al (2021) Utility and futility of skin testing to address concerns surrounding messenger RNA coronavirus disease 2019 vaccine reactions. Ann Allergy Asthma Immunol Off Publ Am Coll. Allergy Asthma Immunol S1081–1206(21)01252–01257
Author information
Authors and Affiliations
Contributions
Conceptualization and writing—original draft: Ferdaous Chahed, Nadia Ben Fredj. Data curation: Hichem Belhadjali, Randa Said El Mabrouk, Donia Ghedira, Khadija Mansour. Supervision writing and review: Nadia Ben Fredj, Najah Ben Fadhel, Karim Aouam, Haifa Ben Romdhane, Amel Chaabane, Zohra Chadli.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The patients in this manuscript have given written informed consent to the publication of their case details (images).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben-Fredj, N., Chahed, F., Ben-Fadhel, N. et al. Case series of chronic spontaneous urticaria following COVID-19 vaccines: an unusual skin manifestation. Eur J Clin Pharmacol 78, 1959–1964 (2022). https://doi.org/10.1007/s00228-022-03399-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03399-8