Abstract
Purpose
Intellectual disability (ID) is a chronic neurodevelopmental condition characterised by limitations in intelligence and adaptive skills with an onset prior to the age of 18 years. People with ID have complex healthcare needs and are more likely than the general population to experience multiple comorbidities and polypharmacy, with subsequent increased risk of adverse medication effects. The aim of this scoping review is to characterise rating scales used to measure adverse effects of medication in people with ID.
Methods
Four online databases (PsycINFO, Medline, Web of Science and OpenGrey) were searched in April 2020. Studies were assessed for inclusion against pre-specified eligibility criteria. Reference lists of included studies were hand searched. Data extraction was carried out by two independent reviewers and key findings were tabulated for consideration. Studies were assessed for quality using the Mixed Methods Appraisal Tool.
Results
The search resulted in 512 unique records, of which fifteen met the inclusion criteria. Fourteen scales were identified. All scales assessed adverse effects of psychotropics only. Of the scales, only one, the Matson Evaluation of Drug Side Effects, which focuses on psychotropic medications, was originally developed for use in a population with ID.
Conclusion
The Matson Evaluation of Drug Side Effects scale appears to be the most reliable and well-researched scale in people with ID. However, a scale which measures adverse effects across multiple medication classes would be valuable for use in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intellectual disability (ID) is a chronic neurodevelopmental condition characterised by limitations in intelligence and adaptive skills with an onset prior to the age of 18 years [1].
People with ID have complex healthcare needs and are 2.5 times more likely to experience multiple health problems [2, 3]. A cross-sectional study of 753 people with ID over age 40 found that 71.2% of participants lived with multiple morbidities. The most prevalent of these include mental illness, gastrointestinal disorders, neurological disorders and ocular disease [3]. People with ID are particularly susceptible to polypharmacy due to the extensive medication regimens needed to manage these conditions [4]. Subsequently, people with ID have a high medication burden, especially from anticholinergics, sedatives and psychotropic medications, including antipsychotics [5,6,7,8,9,10,11]. While first-generation antipsychotics are known to cause a range of extrapyramidal symptoms, such as akathisia, tardive dyskinesia (TD) and dystonia, newer generation atypical antipsychotics produce cardiovascular and metabolic adverse effects [12]. The prevalence of epilepsy in people with ID is approximately 22%, compared to 1.1% in the general population [13,14,15]. Antiepileptic medications are often used by people with ID due to the higher prevalence of epilepsy and for their mood stabilisation effects. However, these medications can cause sedation and drowsiness in addition to producing anticholinergic effects [16]. People who have ID are also more likely to experience drug-related adverse effects [17].
It is not appropriate to extrapolate data on adverse effects of medications for people with ID from the general population. This is due to confounding factors such as existing cognitive disabilities and communication difficulties [18], in addition to evidence suggesting that people with ID may have different susceptibility to medication adverse effects. People with ID have been found to be more likely to suffer from movement related adverse effects from antipsychotics in recent UK evidence [19]. Drug-drug interactions, increased body fat, neurological damage, genetic abnormalities and differences in expression of metabolic enzymes responsible for drug degradation are factors which may contribute to altered effects, and adverse effects, of medications in this population [20,21,22]. These adverse effects can have serious clinical consequences, including hospitalisation [23]. Polypharmacy has also been found to be a predictor for mortality in older adults with ID [24].
There are further unique challenges in the care of people with ID. Verbal and non-verbal individuals struggle with communication from childhood into older age, and healthcare professionals may lack knowledge and skills to effectively interact with people with ID [25,26,27]. This has led to a disparity in the efficacy of receptive and expressive communication between people with ID and healthcare providers [28]. This causes difficulties in obtaining accurate information during medication use reviews and the detection of adverse drug effects, especially for symptoms that are more difficult to identify, for example neurological damage [17, 29]. Failure to detect adverse drug events can contribute to worsening quality of life [30]. Diminished cognitive and language skills make it difficult to interpret subjective symptoms of adverse medication effects and objective symptoms may be misattributed to other health conditions, leading to diagnostic overshadowing and incremental prescribing [31,32,33]. As a result, assessment of adverse medication effects often relies on observable behaviours, which may be direct (involving clinical assessment of the person by a trained healthcare professional), or indirect, by interview with a carer [32].
As a potential solution to these difficulties, adverse effect rating scales for medicines frequently used by people with ID have been investigated in this population. These include scales developed specifically for use in people with ID (e.g. the Matson Evaluation of Drug Side Effects (MEDS) [17, 34,35,36,37,38,39,40]) or modified versions of scales developed for the general population (e.g. the Udvalg for Kliniske Undersøgelser (UKU) Rating Scale [41]). These scales aim to objectively identify cognitive and physical adverse medication effects and determine their severity. While scales alone cannot be used to yield a diagnosis of the adverse medication effect, they can be valuable as a screening tool to improve medication monitoring in a population which can be hindered by difficulties in communication [32, 36].
To date, no clear review of the characteristics or psychometric properties of scales used to assess adverse medication effects in people with ID has been conducted.
This scoping review of the literature aims to characterise the existing rating scales used to measure the adverse effects of medication in people living with ID and assess the best approach for their use in practice.
The objectives of the study are as follows:
-
1.
To determine the adverse medication effect scales available for use in people with ID
-
2.
To review the medication classes included in these scales
-
3.
To identify the types of adverse medication effects recognised in each of the scales; and
-
4.
To explore the robustness of the scales in terms of their psychometric properties, reliability, and validity.
Method
Search strategy
A search of studies which used rating scales to determine adverse effects of medication in people with ID was conducted in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” extension for scoping reviews (PRISMA-ScR) guidelines [42].
The electronic literature databases selected for this review were PsycINFO, Medline, Web of Science and OpenGrey. These databases were searched on 4th and 5th April 2020.
Relevant search terms (keywords) and controlled vocabulary related to the core concepts of the research question were developed with reference to relevant literature [43, 44]. The core concepts were (1) people with ID, (2) medication adverse effects and (3) measures or specific rating scales. The search strategy and specific parameters are summarized in Online Resource 1 of the Electronic Supplementary Material.
Hand searching of bibliographies was also performed on articles selected for inclusion. Forward and backward searches were completed by two independent reviewers in April 2020.
Screening and eligibility
Titles and abstracts were screened for relevance by two reviewers and conflicts were resolved by a third independent party. Selection for more in-depth screening was determined by the inclusion and exclusion criteria outlined in Table 1.
Articles retained from title and abstract screening proceeded to full text screening. Full text screening was performed by two reviewers and disagreements were resolved by a third independent party. Eligibility was determined by the previously described inclusion and exclusion criteria. Data were extracted from the selected studies by two further reviewers and relevant details were tabulated into a concise format for consideration.
Quality assessment
The Mixed Methods Appraisal Tool (MMAT) was used to assess the quality of the studies included in this scoping review [45]. Two reviewers undertook the quality assessment of the studies chosen for inclusion in this scoping review.
Results
Search results
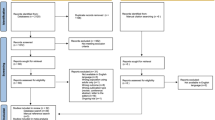
After screening 512 titles and abstracts and 44 full text documents, 15 unique studies across 16 publications were included in the final review, with each article meeting the predetermined inclusion criteria. Figure 1 presents a PRISMA flow diagram of the results.
Characteristics of included studies
All studies evaluated utilised a rating scale to measure adverse effects of medication in a population with ID. All studies were published in the last 20 years and the majority originated in the United States (US) (n = 11), with 8 of these based in the same developmental centres in the South-Eastern US (Louisiana). A choropleth of study location by geographic region is included in Online Resource 2. Most studies were observational in nature (n = 12), while three studies were clinical trials. A brief description of each scale included in the studies discussed is provided in Online Resource 3. Design characteristics of included studies are detailed in Table 2.
Adverse medication effect scales
Fourteen different adverse effect rating scales were identified which measure both physical and cognitive adverse medication effects. Four scales were examined exclusively in children with ID, seven scales were examined exclusively in adults aged 18 years and over, while three scales have been investigated across both populations (Online Resource 4).
Medication classes
The medication classes examined across the studies were predominantly nervous system agents: antipsychotics (11 studies), antiepileptic drugs (7 studies), antidepressants (5 studies), anxiolytics (4 studies), antihypertensives (3 studies), psychostimulants (2 studies) and anticholinergics (2 studies).
Types of adverse medication effects
The scales measured psychotropic medication adverse effects (MEDS, Neurological Side Effect Scale (NSEC), Barkley’s Side Effect Rating Scale (SERS), UKU), more specific movement-related adverse medication effects (Akathisia Ratings of Movement Scale (ARMS), Dyskinesia Identification System Condensed User Scale (DISCUS)), cognitive ability (Hamburg Wechsler Intelligenztest für Erwachsene (HAWIE-R), Five Point Test, Regensburger Wortflüssigkeitstest (RWT), Rivermead Behavioural Memory Test (RBMT), Trail Making Test), stereotyped behaviour (Stereotyped Behaviour Scale), tics (Yale-Brown Global Tic Severity Scale) and obsessive–compulsive symptoms (Yale-Brown Obsessive–Compulsive Scale).
Psychometric properties
One document examined the psychometric properties of two scales, MEDS and ARMS [32]. Both scales were found to be valid, i.e. both scales correctly indicated akathisia in those individuals with a diagnosis of akathisia and did not indicate akathisia in those without a diagnosis. Both scales were also found to be reliable, with high inter-rater agreement and internal consistency.
Quality assessment
All studies included in this scoping review were accepted under the criteria for one of the MMAT categories and were of sufficient quality (Online Resource 5).
Discussion
The accurate and timely detection of adverse effects of medication can significantly impact on quality of life and activities of daily living for people with ID [56]. This study examined the existing literature on medication adverse effect rating scales used in people with ID, the medication classes included in these scales, types of adverse medication effects identified and the available evidence on psychometric properties of these scales. Fifteen studies were deemed eligible for inclusion in the review and fourteen rating scales were identified which assessed the side effects of medications, primarily psychotropics, among people with ID.
Availability of scales
There remains a paucity of research conducted in this field despite the widespread knowledge that people with ID are more susceptible to the detrimental adverse effects of medication due to unique abnormalities in neurological functioning and drug processing [57]. Of the fourteen rating scales identified in the review, only one was specifically developed for use in people with ID—the MEDS scale. Eight studies utilised the MEDS scale or subscale to identify adverse effects of medication. This scale has been described as the most reliable and most researched scale in participants with ID [34]. However, a substantial proportion of the sources which used the MEDS scale, and subsequently their associated results, were obtained through analysis of participants drawn from the same cohort by the same researchers.
Scales investigated in children with ID
Four scales identified in this review were only investigated in participants aged under 18 years. These scales were the Stereotyped Behaviour Scale, Barkley’s SERS, Yale-Brown Global Tic Severity Scale and Yale-Brown Obsessive–Compulsive Scale.
The results obtained from the research of Correia Filho et al. suggest that observations from the two scales used in the study, UKU and Barkley’s SERS, correlate with the adverse medication effect profile observed from the use of risperidone and methylphenidate in the general population with average IQ [47]. The association of methylphenidate with insomnia, decreased appetite, weight loss and gastrointestinal adverse effects has been extensively documented in previous literature concerning the general population [58, 59]. Similarly, research has identified somnolence and weight gain as adverse effects of risperidone use in children with normal IQ [60]. These findings are akin to the results obtained from the sample of children and adolescents with moderate ID in this study. Although in this study the UKU and Barkley’s SERS rating scales were not directly adapted to children with ID, the correlation of results suggests that these scales could have utility for monitoring psychotropic adverse effects in this group. Correia Filho et al. suggest that children with ID presenting with only ADHD symptoms could be initiated on first line treatment with methylphenidate to minimise any unnecessary weight gain associated with risperidone. Conversely, children experiencing weight loss could benefit from initial treatment with risperidone to control symptoms [47]. However, the small sample size of the study limits the robustness of this recommendation.
Efforts have also been made to adapt existing rating scales to be directly applicable to children with ID. Ghuman et al. utilised the developmental disorder version of the Yale-Brown Obsessive–Compulsive Scale to assess the effects of methylphenidate for the treatment of ADHD symptoms in children with ID [50]. This version of the scale was modified to facilitate language difficulties by addressing compulsive behaviour only. Ghuman et al. also employed three additional scales to assess the safety of methylphenidate in the treatment of ADHD in preschool children with ID. The scales chosen reflected established adverse effects of methylphenidate that were hypothesised to affect people with ID. The utilisation of multiple scales enabled the authors to examine a wide range of adverse medication effects in the study sample. The effects of methylphenidate on physical, emotional and social functioning, including movement and stereotypic behaviour, were all considered, factors which could not be assessed collectively through a single scale. Overall, this approach established a greater understanding of the extent of methylphenidate adverse effects for preschool children with ID.
Scales investigated in adults with ID
Seven scales identified in this review were exclusively investigated in participants aged over 18 years. These scales were the ARMS, HAWIE-R, Five-Point Test, MEDS, RWT, RBMT and Trail-Making Test.
Garcia et al. established that the MEDS and ARMS scales were effective in the assessment of symptoms of akathisia in adults with ID [49]. Interestingly, the two scales use different methods of data collection; the MEDS is an interview assessment, whereas the ARMS is an interactive observation assessment. When used in combination, the different methods of assessment allow for greater diagnostic clarity. Moreover, the two scales evaluate somewhat different symptoms, increasing coverage of the range of akathisia symptoms.
In assessing the adverse effects of TD due to the long-term use of atypical antipsychotics in people with ID, both the DISCUS and subscale of the MEDS scale (Central Nervous System-Parkinsonism/Dyskinesia) were utilised by Fodstad et al. The DISCUS scale has been previously shown to have an excellent convergent validity with the Central Nervous System-Parkinsonism/Dyskinesia subscale [48]. The Central Nervous System-Parkinsonism/Dyskinesia subscale was the primary scale utilised in this study. This is possibly due to the Central Nervous System-Parkinsonism/Dyskinesia subscale’s ability to distinguish TD from similarly presenting side-effects, whereas the DISCUS scale has been designed to measure dyskinesia only, rather than a range of side-effects that may be associated with psychotropic medications [35]. Furthermore, parkinsonism and dystonia are additional items that are only included in the MEDS subscale and not the DISCUS scale. These elements are important in assessing the extrapyramidal effects of psychotropic medications. Nonetheless, due to the significant correlation between both scales, both can be beneficial in assessing TD in people with ID.
Scales identified in both children and adults with ID
Three scales identified in this review have been assessed in both adults and children with ID. These scales were the DISCUS, NSEC and UKU.
Hellings et al. employed a rating scale and a checklist, DISCUS and NSEC, to evaluate the adverse medication effects in people taking risperidone vs. placebo both in acute phase and long term treatment [52]. The use of both the scales and the checklist was effective in investigating the various adverse medication effects that were present in participants, with the DISCUS focusing on dyskinesia-related side effects and the NSEC focusing on neuroleptic side effects such as gastrointestinal upset, tremor and urinary incontinence. This allowed for greater insight into adverse effects caused by the medication in comparison to the placebo. The study design, an acute phase of 22 weeks and a 24-week follow up, allowed for the DISCUS and NSEC scales to monitor adverse effects of the drug over both a shorter and longer period of time.
Tveter et al. demonstrated the ability to adjust an established rating scale, making it more applicable to use for adults with ID. In this study, the revised UKU scale consisted of 35 items of the original 48-item scale that can be observed in people with ID [41]. Although it is a condensed scale, little information appears to be lost and adverse medication effects can be evaluated with increased accuracy. This has time and clinical implications in practice for monitoring medication-related adverse effects through observation in people with ID.
Classes of medication
The scales identified in this review were predominantly used to assess adverse effects of psychotropic medication. A significant positive correlation was observed between the use of multiple psychotropic medications across different classes and an increased adverse medication effects profile.
The small, observational study by Matson et al. revealed a significant difference in MEDS severity ratings between individuals prescribed no psychotropics, those prescribed a selective serotonin reuptake inhibitor and those taking a selective serotonin reuptake inhibitor plus additional psychotropics [38]. The participants taking multiple psychotropic agents reported the highest adverse medication effects burden, followed by those exclusively taking a selective serotonin reuptake inhibitor.
These results were mirrored by a second study which emphasised that the more medications administered to people with ID, the greater the risk of untoward, largely irreversible adverse medication effects occurring [54]. This argument was supported by the results of the MEDS scale, which demonstrated that individuals prescribed two psychotropics scored significantly higher in the Central Nervous System-Behavioural/Akathisia subscale than individuals on a single agent. Furthermore, individuals prescribed several psychotropics had significantly higher sores on the Central Nervous System-Parkinsonism/Dyskinesia subscale than either the control or single psychotropic group.
Matson et al. investigated the use of the MEDS scale to assess adverse effects of antipsychotic medications in people with ID with comorbid TD and TD/akathisia [39]. Antipsychotic-related akathisia is well-established, and it is possible that the symptoms of TD and akathisia are heightened by antipsychotic medication [39, 40]. They concluded that people with TD/akathisia experienced an increased number of adverse medication effects than the groups without TD and with TD alone. The clinical significance of these findings has yet to be explored as further research is required with regards to how to manage and treat these adverse medication effects. However, it was emphasised that early detection is the best method for preventing such adverse medication effects.
Scale robustness
Only one study conducted by Garcia et al. in 2006 [32] examined the psychometric validity of the MEDS and ARMS scales. The results from this study provided evidence of criterion validity. This allows the use of both scales in differentiating people with and without akathisia. However, concurrent validity was not demonstrated when both scales were correlated with each other. This could be explained by the fact that there is a variability in presented symptoms, particularly that akathisia symptoms vary in mild cases and over time. The lack of correlation between the scales suggests that both scales model different aspects of the akathisia construct. The most obvious difference is that the MEDS subscale combines both challenging behaviour and akathisia. This is supported by the higher Cronbach’s alpha in the MEDS than the ARMS subscale. One limitation of this paper is that it primarily focused on chronic akathisia and not acute drug-induced akathisia. Nevertheless, the authors concluded that as both scales demonstrated criterion validity but did not demonstrate concurrent validity, this would advise the recommendation for the multitrait-multimethod approach in assessing people with ID. However, multiple factors must be considered, including exposure to psychotropic medications, absence of non-drug causes, baseline behaviour and other movement disorders [32].
Strengths and limitations
This review had many strengths. It is the only review to date, to our knowledge, in which information on all available rating scales used to measure medication adverse effects in people with ID is presented. A recent review by Copeland et al. examined measurement tools for adverse medication effect assessment in people with ID. However, this article focuses exclusively on rating scales designed to assess anti-epileptic adverse effects, as opposed to examining adverse medication effect scales in its entirety [61]. This review adopted a more comprehensive approach, with all medication-related adverse effects scales that are established as applicable, or potentially could be applicable to people with ID with appropriate adaptations, being examined. Inclusion and exclusion criteria were discussed and decided on by eight reviewers. Data extraction was completed by two reviewers and checked for accuracy and completeness by a third reviewer. Additionally, risk of bias was reduced in the screening process as two independent reviewers screened at each stage and disputes were resolved by a third reviewer. Few limits and filters were applied to each database search and all relevant articles from the past 20 years were retrieved. Articles before this time frame would not have much relevance in the current context of medication for people with ID as the landscape has evolved markedly in recent years. Forward and backward searching was performed on the references from included articles which created a more comprehensive representation of available scales. Critical appraisal was carried out using the MMAT. All studies included were deemed to meet the quality criteria outlined and had a low-medium risk of bias.
This review also had several limiting factors. While four major databases were searched for relevant articles, searching of other electronic databases may have resulted in a more comprehensive review. Reviewers were limited in the comparison and categorisation of studies due to the heterogeneity of the studies included. It was not possible to adopt a meta-analytic approach to combine the results of the studies due to variety in study design, outcome measures and outcomes assessed. Elements of PRISMA such as a quantitative assessment of internal biases were not performed as part of this study.
Conclusions and future work
People with ID are often diagnosed with multiple comorbidities and are prescribed complex medication regimens to manage their conditions. It can be difficult for healthcare professionals to recognise adverse medication effects due to the difficulties in communication with this group. This review demonstrates an overall lack of suitable scales in assessing adverse effects of medications across domains in people with ID. The MEDS scale appears to be the most reliable and well researched scale in people with ID. However, when determining the robustness of this scale in comparison with the ARMS, concurrent validity was not demonstrated due to the variability of the symptoms present. Nevertheless, several studies reported the benefit of the use of multiple scales in assessing adverse effects of medications in people with ID. The utilisation of multiple scales provided a greater understanding and holistic approach to adverse medication effect monitoring when implemented. It is evident that more research needs to be carried out to determine the validity of these scales in assessing the adverse effects of medications in people with ID. In addition, the focus on psychotropic medication adverse effects across scales has resulted in a paucity of methods to determine adverse effects from other classes of medication. A scale which measures adverse effects across multiple medication classes would be valuable for use in this population.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington, VA
Heslop P, Blair PS, Fleming P, Hoghton M, Marriott A, Russ L (2014) The Confidential Inquiry into premature deaths of people with intellectual disabilities in the UK: a population-based study. Lancet 383(9920):889–895. https://doi.org/10.1016/s0140-6736(13)62026-7
McCarron M, Swinburne J, Burke E, McGlinchey E, Carroll R, McCallion P (2013) Patterns of multimorbidity in an older population of persons with an intellectual disability: results from the intellectual disability supplement to the Irish longitudinal study on aging (IDS-TILDA). Res Dev Disabil 34(1):521–527. https://doi.org/10.1016/j.ridd.2012.07.029
O’Dwyer M, Peklar J, McCallion P, McCarron M, Henman M (2016) Factors associated with polypharmacy and excessive polypharmacy in older people with intellectual disability differ from the general population: a cross-sectional observational nationwide study. BMJ Open. https://doi.org/10.1136/bmjopen-2015-010505
O’Connell J, Burke E, Mulryan N, O’Dwyer C, Donegan C, McCallion P et al (2018) Drug burden index to define the burden of medicines in older adults with intellectual disabilities: An observational cross-sectional study. Br J Clin Pharmacol 84(3):553–567. https://doi.org/10.1111/bcp.13479
O’Connell J, Henman MC, Burke É, Donegan C, McCallion P, McCarron M et al (2019) Association of drug burden index with grip strength, timed up and go and Barthel index activities of daily living in older adults with intellectual disabilities: an observational cross-sectional study. BMC Geriatr 19(1):173. https://doi.org/10.1186/s12877-019-1190-3
Bowring D, Totsika V, Hastings R, Toogood S, McMahon M (2017) Prevalence of psychotropic medication use and association with challenging behaviour in adults with an intellectual disability. A total population study. J Intellect Disabil Res 61(6):604–17. https://doi.org/10.1111/jir.12359
Sheehan R, Hassiotis A, Walters K, Osborn D, Strydom A, Horsfall L (2015) Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population based cohort study. BMJ 351:h4326
Hove O, Biringer E, Havik OE, Assmus J, Braatveit KJ, Holm SEH et al (2019) Prevalence of drug use among adults with intellectual disabilities compared with drug use in the general population. Pharmacoepidemiol Drug Saf 28(3):337–44. https://doi.org/10.1002/pds.4741
Ward LM, Stanley B, Greenlaw N, Cooper SA, Pacitti C, Henderson A et al (2021) Risk of anticholinergic burden in adults with intellectual disabilities: a Scottish retrospective cohort study of <i>n</i> = 17 220. J Intellect Disabil Res 65(9):813–830. https://doi.org/10.1111/jir.12861
Henderson A, Kinnear D, Fleming M, Stanley B, Greenlaw N, Young-Southward G et al (2021) Antipsychotic and antidepressant prescribing for 704 297 children and young people with and without intellectual disabilities: record linkage study. B J Psych 218(1):58–62. https://doi.org/10.1192/bjp.2020.232
Stroup TS, Gray N (2018) Management of common adverse effects of antipsychotic medications. World Psychiatry 17(3):341–356. https://doi.org/10.1002/wps.20567
Robertson J, Hatton C, Emerson E, Baines S (2015) Prevalence of epilepsy among people with intellectual disabilities: a systematic review. Seizure 29:46–62. https://doi.org/10.1016/j.seizure.2015.03.016
Morgan CL, Baxter H, Kerr MP (2003) Prevalence of epilepsy and associated health service utilization and mortality among patients with intellectual disability. Am J Ment Retard 108(5):293–300. https://doi.org/10.1352/0895-8017(2003)108%3c293:POEAAH%3e2.0.CO;2
Picot MC, Baldy-Moulinier M, Daures JP, Dujols P, Crespel A (2008) The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia 49(7):1230–1238. https://doi.org/10.1111/j.1528-1167.2008.01579.x
O’Dwyer M, Peklar J, Mulryan N, McCallion P, McCarron M, Henman MC (2018) Prevalence and patterns of anti-epileptic medication prescribing in the treatment of epilepsy in older adults with intellectual disabilities. J Intellect Disabil Res 62(3):245–261. https://doi.org/10.1111/jir.12461
Matson JL, Mahan S (2010) Antipsychotic drug side effects for persons with intellectual disability. Res Dev Disabil 31(6):1570–1576. https://doi.org/10.1016/j.ridd.2010.05.005
Kerr M, Scheepers M, Arvio M, Beavis J, Brandt C, Brown S et al (2009) Consensus guidelines into the management of epilepsy in adults with an intellectual disability. J Intellect Disabil Res 53(8):687–694. https://doi.org/10.1111/j.1365-2788.2009.01182.x
Sheehan R, Horsfall L, Strydom A, Osborn D, Walters K, Hassiotis A (2017) Movement side effects of antipsychotic drugs in adults with and without intellectual disability: UK population-based cohort study. BMJ Open 7(8). https://doi.org/10.1136/bmjopen-2017-017406
Winterhalder R (2008). Psychopharmacological Issues. In: Clark LL, Griffiths PRMN, editors. Learning disability and other intellectual impairments: meeting needs throughout health services. John Wiley & Sons, Chichester
de Winter CF, Bastiaanse LP, Hilgenkamp TI, Evenhuis HM, Echteld MA (2012) Overweight and obesity in older people with intellectual disability. Res Dev Disabil 33(2):398–405. https://doi.org/10.1016/j.ridd.2011.09.022
McMahon M, Hatton C, Bowring DL, Hardy C, Preston NJ (2021) The prevalence of potential drug-drug interactions in adults with intellectual disability. J Intellect Disabil Res 65(10):930–940. https://doi.org/10.1111/jir.12844
Zhou M, Du W, Salvador-Carulla L, Glasgow N (2019) Adverse drug event-related hospitalisation in persons with neurodevelopmental disorders: a state-wide retrospective cohort study. J Intellect Disabil Res 63(5):429–40. https://doi.org/10.1111/jir.12586
Schoufour JD, Oppewal A, van der Maarl HJK, Hermans H, Evenhuis HM, Hilgenkamp TIM et al (2018) Multimorbidity and polypharmacy are independently associated with mortality in older people with intellectual disabilities: a 5-year follow-up from the HA-ID study. Am J Intellect Dev Disabil 123(1):72–82. https://doi.org/10.1352/1944-7558-123.1.72
Smith M, Manduchi B, Burke É, Carroll R, McCallion P, McCarron M (2020) Communication difficulties in adults with intellectual disability: results from a national cross-sectional study. Res Dev Disabil 97:103557. https://doi.org/10.1016/j.ridd.2019.103557
Marrus N, Hall L (2017) Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am 26(3):539–554. https://doi.org/10.1016/j.chc.2017.03.001
Hemm C, Dagnan D, Meyer TD (2015) Identifying training needs for mainstream healthcare professionals, to prepare them for working with individuals with intellectual disabilities: a systematic review. J Appl Res Intellect Disabil 28(2):98–110. https://doi.org/10.1111/jar.12117
Lennox NG, Diggens JN, Ugoni AM (1997) The general practice care of people with intellectual disability: barriers and solutions. J Intellect Disabil Res 41(5):380–390. https://doi.org/10.1111/j.1365-2788.1997.tb00725.x
Bhaumik S, Gangadharan SK, Branford D, Barrett (eds) M (2015) The Frith prescribing guidelines for people with intellectual disability. 3rd ed. Wiley Blackwell, United Kingdom
O’Dwyer M, Maidment ID, Bennett K, Peklar J, Mulryan N, McCallion P et al (2016) Association of anticholinergic burden with adverse effects in older people with intellectual disabilities: an observational cross-sectional study. Br J Psychiatry 209:1–7. https://doi.org/10.1192/bjp.bp.115.173971
Bodfish JW, Newell KM, Sprague RL, Harper VN, Lewis MH (1997) Akathisia in adults with mental retardation: development of the Akathisia Ratings of Movement Scale (ARMS). Am J Ment Retard 101(4):413–423
Garcia M (2006) Psychometric validity for the Matson evaluation of drug side effects and the akathisia rating of movement scale. Dissertation, Louisiana State University
O’Dwyer M, McCallion P, McCarron M, Henman M (2018) Medication use and potentially inappropriate prescribing in older adults with intellectual disabilities: a neglected area of research. Ther Adv Drug Saf 9(9):535–557. https://doi.org/10.1177/2042098618782785
Matson JL, Cervantes PE (2013) Current status of the Matson evaluation of drug side effects (MEDS). Res Dev Disabil 34(5):1849–1853. https://doi.org/10.1016/j.ridd.2013.02.030
Matson JL, Fodstad JC, Rivet TT (2008) The convergent and divergent validity of the Matson evaluation of drug side-effects (MEDS) and the dyskinesia identification system: condensed user scale (DISCUS). J Intellect Dev Disabil 33(4):337–344. https://doi.org/10.1080/13668250802478799
Matson JL, Mayville EA, Bamburg JW, Scott Eckholdt C (2001) An analysis of side-effect profiles of anti-seizure medications in persons with intellectual disability using the Matson evaluation of drug side effects (MEDS). J Intellect Dev Disabil 26(4):283–295. https://doi.org/10.1080/13668250120087308
Matson JL, Mayville EA, Bielecki J, Barnes WH, Bamburg JW, Baglio CS (1998) Reliability of the Matson evaluation of drug side effects scale (MEDS). Res Dev Disabil 19(6):501–506. https://doi.org/10.1016/s0891-4222(98)00021-3
Matson JL, Rivet TT, Fodstad JC (2009) Matson evaluation of drug side-effects (MEDS) profiles of selective serotonin reuptake inhibitors (SSRI) in adults with intellectual disability. J Dev Phys Disabil 21(1):57–68. https://doi.org/10.1007/s10882-008-9125-5
Matson JL, Rivet TT, Fodstad JC (2008) Matson evaluation of drug side-effects (MEDS) profiles in adults with intellectual disability, tardive dyskinesia, and akathisia. J Dev Phys Disabil 20(3):283–295. https://doi.org/10.1007/s10882-007-9097-x
Matson JL, Rivet TT, Fodstad JC (2010) Atypical antipsychotic adjustments and side-effects over time in adults with intellectual disability, tardive dyskinesia, and akathisia. J Dev Phys Disabil 22(5):447–461. https://doi.org/10.1007/s10882-009-9179-z
Tveter A, Bakken T, Bramness J, Rossberg J (2014) Adjustment of the UKU side effect rating scale for adults with intellectual disabilities. A pilot study. Adv Ment Health Intellect Disabil 8:260–267. https://doi.org/10.1108/AMHID-11-2013-0064
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169(7):467–473. https://doi.org/10.7326/M18-0850
Sheerin F, Eustace-Cook J, Wuytack F, Doyle C (2021) Medication management in intellectual disability settings: a systematic review. J Intellect Disabil 25(2):242–276. https://doi.org/10.1177/1744629519886184
van Strien AM, Keijsers CJ, Derijks HJ, van Marum RJ (2015) Rating scales to measure side effects of antipsychotic medication: A systematic review. J Psychopharmacol 29(8):857–866
Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P et al (2018) The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Educ Inf 34:285–291. https://doi.org/10.3233/EFI-180221
Brandt C, Lahr D, May TW (2015) Cognitive adverse events of topiramate in patients with epilepsy and intellectual disability. Epilepsy Behav 45:261–264. https://doi.org/10.1016/j.yebeh.2014.12.043
Correia Filho AG, Bodanese R, Silva TL, Alvares JP, Aman M, Rohde LA (2005) Comparison of Risperidone and methylphenidate for reducing ADHD symptoms in children and adolescents with moderate mental retardation. J Am Acad Child Adolesc Psychiatry 44(8):748–755. https://doi.org/10.1097/01.chi.0000166986.30592.67
Fodstad JC, Bamburg JW, Matson JL, Mahan S, Hess JA, Neal D et al (2010) Tardive dyskinesia and intellectual disability: an examination of demographics and topography in adults with dual diagnosis and atypical antipsychotic use. Res Dev Disabil 31(3):750–759. https://doi.org/10.1016/j.ridd.2010.01.017
Garcia MJ, Matson JL (2008) Akathisia in adults with severe and profound intellectual disability: a psychometric study of the MEDS and ARMS. J Intellect Dev Disabil 33(2):171–176. https://doi.org/10.1080/13668250802065190
Ghuman JK, Aman MG, Lecavalier L, Riddle MA, Gelenberg A, Wright R et al (2009) Randomized, placebo-controlled, crossover study of methylphenidate for attention-deficit/hyperactivity disorder symptoms in preschoolers with developmental disorders. J Child Adolesc Psychopharmacol 19(4):329–339. https://doi.org/10.1089/cap.2008.0137
Hellings JA, Zarcone JR, Reese RM, Valdovinos MG, Marquis JG, Fleming KK et al (2006) A crossover study of risperidone in children, adolescents and adults with mental retardation. J Autism Dev Disord 36(3):401–411. https://doi.org/10.1007/s10803-006-0078-1
Hellings JA, Cardona AM, Schroeder SR (2010) Long-term safety and adverse events of Risperidone in children, adolescents, and adults with pervasive developmental disorders. J Ment Health Res Intellect Disabil 3(3):132–144. https://doi.org/10.1080/19315864.2010.494763
Hess J, Matson J, Neal D, Mahan S, Fodstad J, Bamburg J et al (2010) A comparison of psychotropic drug side effect profiles in adults diagnosed with intellectual disabilities and autism spectrum disorders. J Ment Health Res Intellect Disabil 3(2):85–96. https://doi.org/10.1080/19315861003690588
Mahan S, Holloway J, Bamburg JW, Hess JA, Fodstad JC, Matson JL (2010) An examination of psychotropic medication side effects: does taking a greater number of psychotropic medications from different classes affect presentation of side effects in adults with ID? Res Dev Disabil 31(6):1561–9. https://doi.org/10.1016/j.ridd.2010.05.006
Matson JL, Bamburg JW, Mayville EA, Logan JR (2000) Tardive dyskinesia and developmental disabilities: an examination of demographics and topography in persons with dual diagnosis. Br J Dev Disabil 46(91):119–130. https://doi.org/10.1179/096979500799155711
Scheifes A, Walraven S, Stolker JJ, Nijman HLI, Egberts TCG, Heerdink ER (2016) Adverse events and the relation with quality of life in adults with intellectual disability and challenging behaviour using psychotropic drugs. Res Dev Disabil 49–50:13–21. https://doi.org/10.1016/j.ridd.2015.11.017
Goldberg JF, Ernst CL (2016) Core concepts involving adverse psychotropic drug effects: assessment, implications, and management. Psychiatr Clin North Am 39(3):375–389. https://doi.org/10.1016/j.psc.2016.04.001
Barkley RA, McMurray MB, Edelbrock CS, Robbins K (1990) Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 86(2):184–192
Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M et al (2015) Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Sys Rev (11). https://doi.org/10.1002/14651858.CD009885.pub2
Frazier JA, Meyer MC, Biederman J, Wozniak J, Wilens TE, Spencer TJ et al (1999) Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 38(8):960–5. https://doi.org/10.1097/00004583-199908000-00011
Copeland L, Meek A, Kerr M, Robling M, Hood K, McNamara R (2017) Measurement of side effects of anti-epileptic drugs (AEDs) in adults with intellectual disability: a systematic review. Seizure 51:61–73. https://doi.org/10.1016/j.seizure.2017.07.013
Acknowledgements
The authors would like to acknowledge Jessica Eustace-Cook for her advice on search strategy development and execution and contribution to identification of the search terms for intellectual disability. The authors would also like to acknowledge the assistance of Prof Cristín Ryan in preparation of the final manuscript for submission.
Funding
Open Access funding provided by the IReL Consortium. No funding was received for conducting this review.
Author information
Authors and Affiliations
Contributions
All authors contributed in overall conception and design of the review. All authors contributed to development of search strategy, including selection of appropriate databases and search terms. NK and RMcL completed database searches; AK and CL completed forward searches; OM and RMcL completed backward searches. MT, NK and OM completed title and abstract screening; disagreements were resolved by consensus with LM. KL and LM completed full-text screening; disagreements were resolved by consensus with NK and MT. CL and KL applied the MMAT appraisal tool. LM and AK analysed findings. NK, AK, KL, CL, RMcL, LM, OM and MT wrote the first draft of the manuscript; JO’C and KR revised the manuscript. JO’C is the publication guarantor.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Neasa Kelly, Andrew Kilmartin, Kevin Lannon, Caren Lee, Rory McLoughlin, Lara Mulvanny, Omnyiah Mohamed, and Mairead Treacy are joint first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kelly, N., Kilmartin, A., Lannon, K. et al. Rating scales to measure adverse effects of medications in people with intellectual disability: a scoping review. Eur J Clin Pharmacol 78, 1711–1725 (2022). https://doi.org/10.1007/s00228-022-03375-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03375-2