Abstract
Background
Although statin use is reported to decrease after dementia diagnosis, time to statin discontinuation and factors associated with discontinuation have not been studied in persons with Alzheimer’s disease (AD). We compared the risk of discontinuation and factors associated with discontinuation, including secondary and primary prevention indication, in statin users with and without AD.
Methods
The register-based Medication Use and Alzheimer’s Disease (MEDALZ) cohort includes community dwellers with a clinically verified AD diagnosed during 2005–2011 in Finland. On the AD diagnosis date (index date), each person with AD was matched with a comparison person without AD. We included 25,137 people with AD and 22,692 without AD who used statin on the index date or initiated within 90 days after. Cox regression models restricted to 4-year follow-up were conducted.
Result
The median time to statin discontinuation was 1.46 years in people with AD and 1.36 years in people without AD. People with AD were more likely to discontinue than people without AD (adjusted HR (aHR) 1.20 (95% CI 1.18–1.24)). This was observed for both primary (aHR 1.11 (1.06–1.16)) and secondary prevention (aHR 1.30 (1.25–1.35)) purpose. Factors associated with discontinuation included higher age and female gender, whereas concomitant cardiovascular drug use and previous statin use were associated with decreased risk.
Conclusion
The absolute difference in discontinuation rates was small, and the same factors were associated with statin discontinuation in people with and without AD. The findings suggest that cognitive decline plays a minor role on statin discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Statins are used for prevention of atherosclerotic cardiovascular diseases. However, there have been doubts about statin efficacy in older persons, especially when used for primary prevention [1]. Previous studies have showed that the prevalence of statin use declines after dementia/Alzheimer’s disease (AD) diagnosis [2, 3]. An Australian study of persons over 65 years with dementia observed that over a half of them (58.7%) discontinued statin use during a 3-year follow-up [4]. In a United Kingdom (UK) cohort study conducted in primary care, long-term statin users with dementia were more likely to discontinue statins than people without dementia, regardless of whether statins were prescribed for primary or secondary prevention [5]. In a Danish study of persons over 70 years, 33% long-term users with dementia discontinued statin therapy [6].
Although the more common discontinuation in people with dementia has consistently been reported, it is unknown when statins are discontinued in persons with AD and which characteristics, including primary or secondary prevention, are associated with discontinuation. Hence, the purpose of our study was to investigate the time to statin discontinuation from AD diagnosis and to compare the risk of statin discontinuation and associated factors in persons with and without AD.
Methods and material
The Medication Use and Alzheimer’s Disease study
Study was conducted on the MEDALZ (Medication Use and Alzheimer’s Disease) study. MEDALZ includes 70,718 community-dwelling people who got a clinically verified AD diagnosis during 2005–2011 [7] and a matched comparison cohort without AD. The Special Reimbursement Register, maintained by the Social Insurance Institution of Finland (SII), was used to identify persons with AD. This register also contains information on reimbursement according to specific chronic diseases such as diabetes, cardiovascular diseases, and Alzheimer’s disease. This study utilized data from Prescription Register, the Special Reimbursement Register, the Care Register for Health Care, and Statistics Finland.
The AD diagnosis is consistent with criteria of NINCDS–ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association) and DSM-IV criteria for AD (Diagnostic and Statistical Manual Fourth edition) [8, 9] including computed tomography or magnetic resonance, exclusion of alternative diagnosis, and confirmation of diagnosis by a geriatrician or neurologist. Hence, at the time of AD-diagnosis, the MEDALZ cohort contains persons from mild to moderate stages of AD.
Each person in the AD cohort was matched with one comparison person without AD by age (± 1 year), sex, and region of residence at the date of AD diagnosis. The comparison persons were identified from the Social Insurance Institution of Finland database including all residents. To fulfill the criteria of matching, those persons had to meet criteria including (a) alive and community-dwelling on the last day of the month when case was diagnosed with AD, and (b) no special reimbursement for AD medication or acetylcholinesterase inhibitor or memantine purchase before or within 12 months after matching date. The matching date was assigned as index date for these comparison persons.
Identification of statin users
Statin users were identified from Prescription Register with ATC code C10AA. Statin treatment episodes were constructed by AdhereR package of R [10] which calculates duration of use based on purchase dates, assumed or prescribed daily dose (in tablets per day), and with allowed gap (grace period) defined by the investigator. We assumed that statins were used 1 tablet per day [11]. Because we do not have information of drug use during stay in hospital/nursing home, we censored statin users who had hospital stay longer than 60 days to minimize misclassification and the risk of exposure misclassification. A sensitivity analysis was performed with censoring to < 30-day stays.
This study included people who had a treatment episode ongoing on the date of AD diagnosis or the corresponding (index date for persons without AD) or who initiated statin use day within 90 days after the index date. (Supplementary Fig. 1).
Cohort entry date was defined as the date AD diagnosis or the first date of the first statin purchase that began within 90 days after the index date.
Statin discontinuation
Discontinuation was defined as not filling a statin prescription during the days’ supply of the previous dispensing plus grace period. Grace period is an allowed gap which is added to the drug use duration to deal with non-perfect adherence and other variation in purchasing behavior. We used a 120-day grace period to capture true discontinuation. In Finland, medication can be dispensed for 90 days (maximum of 100 days) of treatment at the time and grace period reflects this.
The maximum follow-up time was restricted to 4 years to ensure all people in cohort had the same possible observation period. People were followed up until discontinuation of statin use, and censored to death, over 60 days of hospitalization, end of follow-up (4 years or December 31, 2015) or date when persons without AD were diagnosed with AD, whichever came first.
Covariates
Statin use was categorized as for primary and secondary prevention. Secondary prevention was defined using ICD-10 codes for cardiovascular (CV) diseases including coronary artery disease, coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), ischemic strokes, and atherosclerosis of any neck or brain arteries. Primary prevention was defined as having no conditions defined as secondary prevention. Diagnosis codes, data sources, and time periods are defined in Supplementary Table 1.
Data on comorbidities (chronic heart failure, atrial fibrillation, and diabetes) were extracted from the Special Reimbursement and Care Register for Health Care (including hospital discharges and specialized healthcare outpatient visit) based on ICD 10 (Supplementary Table 2).
A number of cardiovascular drug substances other than statins were extracted from the Prescription Register data with ATC code C* excluding C01C, C04, C05, C10AA. The prevalence of cardiovascular drug substances was identified in 120-day period before and after the index date because this time window matched with our grace period definition and should capture all regular users. The number of cardiovascular drugs substances used was calculated by taking into account on the actual number of drug substances from combination products.
Statistical analysis
We performed descriptive statistics as means, standard deviations (SD), median (interquartile range (IQR)), or frequency and percentages where appropriate. We applied T test for continuous variables with normal distribution, Mann–Whitney U test for continuous variables with skewed distribution, and chi-square test for categorizing variable to compare characteristics between groups. We presented results with 95% confidence intervals.
We used Cox regression models to compare the risk of statin discontinuation between people with and without AD and to assess factors related to statin discontinuation in persons with and without AD. The results were adjusted for age at cohort entry, sex, statin use before cohort entry, indication, sum of cardiovascular drug substances, diabetes, atrial fibrillation, heart failure, calendar year, hospital district. The proportionality assumption was confirmed with Kaplan–Meier curves.
To assess factors associated with statin discontinuation, the same analyses were performed between persons with and without AD and stratified based on primary/secondary prevention and AD status.
To evaluate whether the results were affected by choice of grace period and maximum allowed length of hospital stay, sensitivity analyses were performed with grace period of 90 days and by censoring to 30-day hospital stays.
To illustrate temporal trends in the cohort, the proportions of statin users of each annual cohort (per AD diagnosis year), as well as the cumulative survival (i.e., continuation of statin use), are presented as supplementary analyses.
All statistical analyses were performed using the software R 4.0 program [12] and STATA 14 (Stata Corporation, College Station, TX, USA).
Results
Characteristics of statin users in both cohorts
Altogether, 25,137 persons with AD and 22,692 persons without AD used statin on the index date (date of AD diagnosis) or initiated statin within 90 days after the index date (Table 1). The mean age on cohort entry was approximately 79 years, and majority of persons in both cohorts were women. Statin users with AD had higher prevalence of diabetes, atrial fibrillation, and chronic heart failure than persons without AD.
During the 4-year follow-up, 39.5% of people with AD and 34.7% in of those without AD discontinued statin use (Table 1). The median time from the cohort entry date to the date of discontinuation was similar in both cohorts: 1.46 years in persons with AD and 1.36 years in persons without AD. In both cohorts, over half of persons (55%) had a secondary prevention indication for statin use. Over 90% of people used statins already before cohort entry and over 80% used at least one other cardiovascular drug.
Rates of statin discontinuation
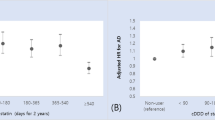
The rate of statin discontinuation was 4.35/10000 person-years in persons with AD and 3.28/10000 person-year in those without AD (Table 2). The relative risk for discontinuation in people with AD was 20% higher than in people without AD (adjusted hazard ratio (aHR) 1.20, 95% CI 1.18–1.24). The relative difference between AD and comparison group was slightly larger in secondary prevention (aHR 1.30, 95% CI 1.25–1.35) than in primary prevention (aHR 1.11, 95% CI 1.06–1.16).
In sensitivity analyses with censoring to 30-day hospitalization, the difference between people with and without AD was similar to that in the main analyses (Table 2). In sensitive analyses with shorter (90 days) grace period, the difference between people with and without AD became larger (80% higher relative risk).
Factors associated with statin discontinuation between people with and without AD
The same characteristics were associated with statin discontinuation in people with and without AD (Table 3). Discontinuation was less common in men than in women and most common in age groups over 75 years or over in people with AD and in age groups 70 years or over in people without AD. The risk of discontinuation was not different between primary and secondary prevention indication users, or those with and without diabetes or atrial fibrillation (Table 3). Heart failure was weakly associated with risk of discontinuation. The risk of discontinuation was lower among users who used higher number of other cardiovascular drugs, or who had used statins before the cohort entry. The association between duration of previous statin use was stronger in people without AD. In both cohorts, statin discontinuation was more common in those with later cohort entry years in comparison to those with index date in 2005 (Table 3, and Supplementary Fig. 2).
Discussion
To our knowledge, this is the first study comparing the statin discontinuation risk and factors associated with discontinuation between people with and without AD. Although people with AD had higher relative risk of statin discontinuation than people without AD during the 4-year follow-up, the absolute difference in discontinuation rates was small. The same factors were associated with discontinuation in people with and without AD, as older people and women were more likely to discontinue whereas users of other cardiovascular drugs and those who had used statins for longer time before the index date were less likely to discontinue in both cohorts. Discontinuation was equally common in primary and secondary indication in both cohorts.
Most of the previous studies on statin discontinuation in people with dementia have applied new user design [4, 5, 13] and only one study included prevalent long-term statin users [6]. The proportion of people with dementia who discontinued in these previous studies has ranged between 33 [6] and 59% [4], with the smallest proportion being observed in the study of long-term statin users. The proportion of people who discontinued in our study is comparable with the earlier Danish study of long-term users [6], although direct comparisons are not meaningful due to differences in study design. Our study included both prevalent users and those who initiated within 90 days of AD diagnosis, with the majority being prevalent users. In the previous studies, discontinuation was more common in people with dementia in the study with long-term statin users [6] while the opposite association was observed in studies conducted among incident short-term users [5, 13].
In our study, statin discontinuation was relatively common in both cohorts and the highest HRs were observed in the oldest age groups. This finding is in line with previous studies [6, 13, 14]. In a Danish study, the difference in the discontinuation between age groups 70–74 years and those aged > 95 years was twofold (aOR = 2.06, 95% CI 1.35–3.16) at 1 year and nearly fourfold at 4 years (aOR3.94, 95% CI 1.83–8.49) of follow-up [13]. A third of participants in our study were aged over 80 years and nearly fifth were at least 85 years old which may partly explain the same kind of risk of discontinuation in both cohorts. In addition, the oldest persons are at the higher risk of statin-related adverse effects [15], and the health status is more unstable than in younger persons, which may explain the higher discontinuation rates among the older participants in our and earlier studies. The changes in health status may also have led to deprescribing. Other comorbidities or progression of disease which negatively affect life expectancy such as cancer [16] could also affect decision of statin deprescribing [17]. In addition, frailty, which is common among older persons with high age and even more common in persons with AD [18], might increase the decision to deprescribe [19]. Although regular medication reviews are recommended by the Finnish authorities, those recommendations are not always applied in clinical practice; therefore, we do not expect that statin discontinuation rates in our study were significantly affected by regular reviews.
Time period–related trends have been observed in the use of statins among older persons aged over 79 years in USA that the proportion of statin users increased from the year 1999 until 2009 in secondary prevention and until 2007 in primary prevention, then decreased after that [20]. Similarly, there was an increase in prevalence of statin use between years 2008 and 2010 among people aged 65 years in Finland and prevalence remained at the same level after that to the end of year 2015 [21]. Consistent with this, we observed an increase in the prevalence of statin use per diagnosis year until 2011, while the discontinuation rate was also higher in those who entered the cohort in later years. Public discussion on whether statins should be used for other than secondary prevention indications [22] and relatively high drug prices but low reimbursement together with time trends described may have impacted our study results. Only a small proportion of statin users in Finland reported to have discontinued statin therapy due to worrying or experienced of side effects [23].
Our results showed that discontinuation was not different due to primary versus secondary prevention in both people with and without AD, which is in line with Danish study [6]. It could be due to consideration of clinicians in the period when benefits of statin in primary prevention were still debated [24]. However, the discontinuation risk was lower among users of other cardiovascular drugs. It could be partly because we may not have captured all milder cardiovascular diseases and consequently in the primary prevention group may include persons with mild coronary disease or atherosclerosis of other arteries. Therefore, it is possible that the number of other cardiovascular drugs better describes the presence and severity of cardiovascular diseases than our diagnosis-based measure of secondary versus primary prevention. Besides, using statin in long period increases adherence to statin use which partially explained the necessaries of statin therapy and stronger commit to therapy in these cases [25].
The slightly higher risk of discontinuation in persons with AD in our study, observed in both primary and secondary prevention indication, may also be due to lower adherence in persons with cognitive decline [26,27,28] that is also reported in Finland [29]. However, for persons with dementia, caregiver or home care services often take care of medications instead of the patient and in this kind of situation, adherence does not describe the behavior of the patient. The relative risk of statin discontinuation in our study is comparable to findings from a systematic review that reported 18% higher risk of statin discontinuation in persons with dementia compared to those without dementia [14].
Strengths and limitations
Use and linkage of different registers from a country with public healthcare system allowed us to perform a nationwide study with low risk of selection bias. We used Prescription Register which captures dispensed medication; thus, the time people redeemed prescription was more precise than prescriptions. Moreover, we use ATC code C10AA which captured statins in general thus accounting for switches into another statin.
However, our study has some limitations. Firstly, this is a register-based study; thus, like other studies, we do not know whether discontinuation decision is made by prescriber (i.e., deprescribing) or by the caregiver or patient and we do not know whether medications were actually taken by patients. We also lack information about changes in severity of comorbidities during the follow-up, which could impact on discontinuation of statin therapy in both cohorts. We also lacked data on patient-related factors such as income that has been previously linked to discontinuation [30]. Due to lacking indication in register data, we may have misclassified some people into primary prevention category. Therefore, further research is needed on the reasons of statin discontinuation, as well as by who and how the decision of statin discontinuation is initiated and made.
Conclusion
Discontinuation was common in both groups and the absolute difference in statin discontinuation rates was small between people with and without AD. These findings suggest that cognitive decline does not have a large impact on discontinuation of statins in older persons.
Availability of data and material
The authors are unable to openly share the data used to conduct this study. The data that support the findings of this study are available from the corresponding author upon reasonable request and with permission of the register maintainers.
References
Armitage J, Baigent C, Barnes E et al (2019) Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 393(10170):407–415. https://doi.org/10.1016/S0140-6736(18)31942-1
Vu M, Koponen M, Taipale H et al (2019) Prevalence of cardiovascular drug use before and after diagnosis of Alzheimer’s disease. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2019.09.036
Picton L, Bell JS, George J, Korhonen MJ, Ilomäki J (2021) The changing pattern of statin use in people with dementia: a population-based study. J Clin Lipidol 15(1):192–201. https://doi.org/10.1016/j.jacl.2020.11.008
Ofori-Asenso R, Ilomaki J, Tacey M et al (2018) Prevalence and incidence of statin use and 3-year adherence and discontinuation rates among older adults with dementia. Am J Alzheimers Dis Other Demen 33(8):527–534. https://doi.org/10.1177/1533317518787314
Vinogradova Y, Coupland C, Brindle P, Hippisley-Cox J (2016) Discontinuation and restarting in patients on statin treatment: prospective open cohort study using a primary care database. BMJ. https://doi.org/10.1136/bmj.i3305
Thompson W, Jarbøl DE, Nielsen JB, Haastrup P, Pottegård A (2020) Statin use and discontinuation in Danes age 70 and older: a nationwide drug utilisation study. Age Ageing. https://doi.org/10.1093/ageing/afaa160
Tolppanen A-M, Taipale H, Koponen M et al (2016) Cohort profile: the Finnish Medication and Alzheimer’s disease (MEDALZ) study. BMJ Open 6(7):e012100. https://doi.org/10.1136/bmjopen-2016-012100
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):263–269. https://doi.org/10.1016/j.jalz.2011.03.005
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939–944. https://doi.org/10.1212/wnl.34.7.939
Dima AL, Dediu D (2017) Computation of adherence to medication and visualization of medication histories in R with AdhereR: towards transparent and reproducible use of electronic healthcare data. PLoS ONE 12(4):1–14. https://doi.org/10.1371/journal.pone.0174426
Romppainen T, Rikala M, Aarnio E, Korhonen MJ, Saastamoinen LK, Huupponen R (2014) Measurement of statin exposure in the absence of information on prescribed doses. Eur J Clin Pharmacol 70(10):1275–1276. https://doi.org/10.1007/s00228-014-1737-3
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org
Thompson W, Jarbøl DE, Haastrup P, Nielsen JB, Pottegård A (2019) Statins in older Danes: factors associated with discontinuation over the first 4 years of use. J Am Geriatr Soc 67(10):2050–2057. https://doi.org/10.1111/jgs.16073
Ofori-Asenso R, Jakhu A, Curtis AJ et al (2018) A systematic review and meta-analysis of the factors associated with nonadherence and discontinuation of statins among people aged ≥65 years. Journals Gerontol - Ser A Biol Sci Med Sci 73(6):798–805. https://doi.org/10.1093/gerona/glx256
Banach M, Rizzo M, Toth PP et al (2015) Statin intolerance - an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci 11(1):1–23. https://doi.org/10.5114/aoms.2015.49807
Todd A, Al-Khafaji J, Akhter N et al (2018) Missed opportunities: unnecessary medicine use in patients with lung cancer at the end of life – an international cohort study. Br J Clin Pharmacol 84(12):2802–2810. https://doi.org/10.1111/bcp.13735
Thompson W, Jarbøl D, Nielsen JB, Haastrup P, Pedersen LB (2021) GP preferences for discussing statin deprescribing: a discrete choice experiment. Fam Pract 10:1–6. https://doi.org/10.1093/fampra/cmab075
Kojima G, Liljas A, Iliffe S, Walters K (2017) Prevalence of frailty in mild to moderate Alzheimer’s disease: a systematic review and meta-analysis. Curr Alzheimer Res. https://doi.org/10.2174/1567205014666170417104236
Gulliford M, Ravindrarajah R, Hamada S, Jackson S, Charlton J (2017) Inception and deprescribing of statins in people aged over 80 years: cohort study. Age Ageing 46(6):1001–1005. https://doi.org/10.1093/ageing/afx100
Johansen ME, Green LA (2015) Statin use in very elderly individuals, 1999–2012. JAMA Intern Med 175(10):1715. https://doi.org/10.1001/jamainternmed.2015.4302
Lonka V, Taipale H, Saastamoinen LK, Kettunen R, Hartikainen S (2017) Kaikkein iäkkäimmät käyttävät statiineja yhä yleisemmin. Suom Lääkäril 42:2363–2368
Ray KK, Seshasai SRK (2010) Statins and all-cause mortality in high-risk primary prevention. Clin Gov An Int J 15(4):15–18. https://doi.org/10.1108/cgij.2010.24815dae.010
Silvennoinen R, Turunen JH, Kovanen PT, Syvänne M, Tikkanen MJ (2017) Attitudes and actions: a survey to assess statin use among Finnish patients with increased risk for cardiovascular events. J Clin Lipidol 11(2):485–494. https://doi.org/10.1016/j.jacl.2017.02.013
Stone NJ, Robinson JG, Lichtenstein AH et al (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the american college of cardiology/american heart association task force on practice guidelines. Circulation 129(25 SUPPL. 1):1–45. https://doi.org/10.1161/01.cir.0000437738.63853.7a
Korhonen MJ, Helin-Salmivaara A, Huupponen R (2011) Dynamics of long-term statin therapy. Eur J Clin Pharmacol 67(9):925–931. https://doi.org/10.1007/s00228-011-1019-2
Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J (2002) Long-term persistence in use of statin therapy in elderly patients. J Am Med Assoc 288(4):455–461. https://doi.org/10.1001/jama.288.4.455
Marcum ZA, Walker RL, Jones BL et al (2019) Patterns of antihypertensive and statin adherence prior to dementia: findings from the adult changes in thought study. BMC Geriatr 19(1):1–10. https://doi.org/10.1186/s12877-019-1058-6
Mizokami F, Mase H, Kinoshita T, Kumagai T, Furuta K, Ito K (2016) Adherence to medication regimens is an effective indicator of cognitive dysfunction in elderly individuals. Am J Alzheimers Dis Other Demen 31(2):132–136. https://doi.org/10.1177/1533317515598859
Aarnio EJ, Martikainen JA, Helin-Salmivaara A et al (2014) Register-based predictors of adherence among new statin users in Finland. J Clin Lipidol 8(1):117–125. https://doi.org/10.1016/j.jacl.2013.09.008
Mann DM, Woodward M, Muntner P, Falzon L, Kronish I (2010) Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother 44(9):1410–1421. https://doi.org/10.1345/aph.1P150
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital. M.V. was funded by the University of Eastern Finland.
Author information
Authors and Affiliations
Contributions
M.V., R.K., S.H., A.M.T., and H.T. planned the study. M.V. and H.T. had full access to all the data in the study. H.T. prepared data. M.V. performed statistical analyses and took responsibility for the integrity of the data and the accuracy of the data analysis. M.V drafted the manuscript. All authors contributed to the interpretation of the data, revised the manuscript, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethic approval
All data were de-identified before sending to research team, and participants were not contacted; therefore, according to Finnish legislation, ethic committee approval was not required.
Conflict of interest
H.T has participated in research projects funded by grants from Janssen-Cilag and Eli Lilly, with grants paid to the employing institution. H.T reports personal fees from Janssen-Cilag and Otsuka. S.H. has got lecture fee from Astellas Pharma (outside this study). A.M.T acknowledges a research grant from Amgen, paid to the institution where she is employed (outside of the submitted work). M.V. and R.K. have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vu, M., Kettunen, R., Tolppanen, AM. et al. Statin discontinuation in persons with and without Alzheimer’s disease. Eur J Clin Pharmacol 78, 1145–1153 (2022). https://doi.org/10.1007/s00228-022-03320-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03320-3