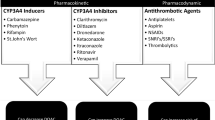
Abstract
Purpose
This study aimed to estimate the prevalence, contributory factors, and severity of medication errors associated with direct acting oral anticoagulants (DOACs).
Methods
A systematic review and meta-analysis were undertaken by searching 11 databases including Medline, Embase, and CINHAL between January 2008 and September 2020. The pooled prevalence of errors and predictive intervals were estimated using random-effects models using Stata software. Data related to error causation were synthesised according to Reason’s accident causation model.
Results
From the 5205 titles screened, 32 studies were included which were mostly based in hospitals and included DOAC treatment for thromboembolism and atrial fibrillation. The proportion of study population who experienced either prescription, administration, or dispensing error ranged from 5.3 to 37.3%. The pooled percentage of patients experiencing prescribing error was 20% (95% CI 15–25%; I2 = 96%; 95% PrI 4–43%). Prescribing error constituted the majority of all error types with a pooled estimate of 78% (95%CI 73–82%; I2 = 0) of all errors. The common reported causes were active failures including wrong drug, and dose for the indication. Mistakes such as non-consideration of renal function, and error-provoking conditions such as lack of knowledge were common contributing factors. Adverse events such as potentially fatal intracranial haemorrhage or patient deaths were linked to the errors but causality assessments were often missing.
Conclusions
Despite their favourable safety profile, DOAC medication errors are common. There is a need to promote multidisciplinary working, guideline-adherence, training, and education of healthcare professionals, and the use of theory-based and technology-facilitated interventions to minimise errors and maximise the benefits of DOACs usage in all settings.
Protocol
A protocol developed as per PRISMA-P guideline is registered under PROSPERO ID = CRD42019122996
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Direct-acting non-vitamin K antagonist oral anticoagulants (DOACs) including direct thrombin-inhibitor dabigatran, and two-factor Xa inhibitors rivaroxaban and apixaban have become the preferred choice in clinical practice for the primary and secondary stroke prevention in patients with atrial fibrillation, prevention and treatment of venous thromboembolism (VTE), and thromboprophylaxis in patients undergoing total hip or knee arthroplasty. Depending on indications, anticoagulation therapy can be given for a short term (up to around three months) or long term. Short-term anticoagulation therapy is most commonly indicated for primary perioperative prophylaxis of thromboembolic events such as those undergoing hip or knee replacement surgery. Long-term anticoagulant therapy with DOACs is recommended primarily for major cardiovascular conditions such as non-valvular atrial fibrillation (NVAF) [1].
DOACs have the advantage over Vitamin-K antagonists (VKA) of a much wider therapeutic window. While the relative ease of prescribing DOACs compared to VKAs makes them more commonly prescribed, therefore, healthcare professionals need to be aware of unwanted treatment outcomes associated with medication errors and suboptimal prescribing [1]. DOACs’ relatively shorter history of use precludes the current availability of long-term safety data. In addition, pharmacokinetic profiles and drug interactions are also not fully understood.
A risk and benefit profiling should be carefully considered/conducted before the prescribing of DOACs. The NICE guideline on atrial fibrillation recommends that bleeding risk, estimated using the HAS-BLED score, is taken into account when offering anticoagulation [1]. The HAS-BLED score estimates the risk of bleeding on a 9-point scale. Dose adjustments are often recommended with DOACs for renal creatinine clearance including contraindications for severe renal impairment [2].
Evidence of adverse events, particularly the incidence of bleeding related to the use of DOACs compared with vitamin-K antagonists in real-life patients, are beginning to emerge [3]. Previous systematic reviews of randomised controlled trials (RCTs) and observational studies have demonstrated the clinical benefits of DOACs compared to VKAs. DOACs significantly reduce the risks of intracranial haemorrhage, gastrointestinal, and major bleeding [4]. However, no direct head-to-head comparisons have been reported for each medicine of DOAC class. This leads to difficulty in the choice of drugs and dosage. A particular challenge is also the lack of availability of specific antidotes for all DOACs, and the lack of clear guidelines around treatment options for patients with intracranial bleeding and gastrointestinal bleeding under DOAC therapy [5].
Considering the above factors, DOACs are known to be one of the most common drug classes that are associated with adverse drug events (ADEs) [6]. The lack of long-term clinical experiences and the need for careful consideration of risk and benefit profiles makes DOAC candidates for medication errors, particularly prescribing errors which are often responsible for such ADEs [6].
To date, there exists no systematic reviews and meta-analysis that synthesise the prevalence of medication errors associated with DOACs including the prevalence of different types of medication errors. In addition, it is imperative to synthesise the contributory factors reported in the literature. Theoretical models are useful in identifying and interpreting factors that contribute to errors and to enable future interventions that can be effective in minimising such errors [7].
Reason’s accident causation model classifies errors into three different categories including (a) active failures which are unsafe acts committed by persons who are in direct contact with the patient or system and includes slips and lapses (errors in task execution), mistakes (errors in planning), and procedural violations (rule breaking); (b) error-provoking conditions within the workplace (e.g., time pressure, understaffing, inadequate equipment, fatigue, and inexperience); and (c) latent failures which arise from decisions made by policy makers, leaders, and top-level management [8]. This model has been widely adopted in research identifying the prevalence and causes of medication errors [9].
This systematic review aimed to determine the prevalence of medication errors associated with DOACs in clinical practice and to identify contributory factors associated with DOACs in adult patients using the Reason’s accident causation model. Results can enable healthcare professionals in diverse settings including primary, secondary, ambulatory care, and those in the interface such as community pharmacy to understand and mitigate common errors and associated consequences on patients and health systems.
Methods
The systematic review protocol was developed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines and registered with the International Prospective Register of Systematic Reviews (PROSPERO code is CRD42019122996). The review is reported according to the PRISMA guidelines [10] and MOOSE statements (Electronic supplementary material 1).
Search strategy
A systemic search of the literature was undertaken using electronic databases: Medline, Embase, and Cumulative Index of Nursing and Cumulative Allied Health Literature (CINAHL), British Nursing Index (BNI), International Pharmaceutical Abstract (IPA), Cochrane Central Register of Controlled Trials (CENTRAL), and grey sources including Institute for Safe Medication Practices (ISMP), The FDA Safety Information and Adverse Event Reporting Program (FDA MedWatch), and Google Scholar databases from 2008 to September 2020. The search terms used included Medical Subject Headings and Natural Language Keywords for DOACs, namely dabigatran, rivaroxaban betrixaban, apixaban, and edoxaban and medication errors (specifically prescribing, and dispensing), and administration errors. Besides, each database search was restricted to English published studies (Electronic supplemental material 2) and conducted using the Boolean operators (AND, OR, and/or NOT). In addition, reference lists of included studies were screened to identify any additional relevant studies.
Types of studies
We included studies which reported or investigated the rate of prescribing, administration, or dispensing errors associated with DOACs. Studies of adverse drug events that are not classified as errors were excluded, as were review articles, letters, opinion papers, and editorials.
Types of participants
Adult patients (≥ 18 years) prescribed DOACs were eligible for inclusion.
Outcomes
The primary outcome was the prevalence of medication errors associated with DOACs. Prevalence data on each error type i.e. prescribing, dispensing, and administration error was obtained by calculating the number of patients for whom an error was identified amongst the total number of patients included during the data collection period. The errors included in this study were those identified from the following source: chart review, medication review, and those reported to the error reporting systems. The secondary outcomes included the nature of causes, contributory factors, and severity of medication errors associated with DOACs.
Study selection and data extraction
Two reviewers (AA and MHA) independently screened the titles and abstracts of all potentially relevant papers based on the selection criteria. This was followed by a full-text screening using Rayyan QCRI (a web and mobile app for a systematic review screening that facilitates collaboration between different reviewers for inclusion and exclusion of studies) [11]. Any disagreement about study inclusion was resolved through discussion with a third reviewer (VP). Duplicate independent extraction of data was undertaken by researchers working in pairs (AA, MHA, VP, ZJ). The data extracted included authors, year of publication, country and setting, study design, error prevalence, the nature of errors, error severity, and contributory factors. Data on study characteristics, error prevalence, and error causes were extracted. A meta-analysis was performed using Stata/IC 15.1 Software (StataCorp, College Station, TX, USA).
Assessment of methodological quality
Quality assessment was undertaken by two independent reviewers (AA and MA) with disagreements resolved by consensus or referred to two other reviewers (HA, VP) as required using the critical appraisal skills programme (CASP) checklist [12].
Data synthesis and statistical analysis
The meta-analyses were performed on the prevalence of medication errors associated with DOACs by a statistician (MP). A random-effects model was used to synthesise the data due to the expected heterogeneity between included studies, and the results obtained were presented using forest plots. Heterogeneity was described using I2 statistics and reporting of 95% prediction intervals [13]. The statistical significance of I2 was tested with chi-square test, and P-value level < 0.05 was set as the level of statistical significance. The effect size was calculated as the proportion with 95% confidence interval (CI).
Data on error causation were synthesized using Reason’s accident causation model [14] as a theoretical framework as per the classification of active failures, error-producing conditions, and latent failures.
Results
Search and study selection
The initial search resulted in 5205 titles search results across all the databases accessed (Fig. 1 shows the PRISMA flow diagram for this study). The duplicate results and studies that did not meet the inclusion criteria were identified and excluded. Overall, 408 articles were assessed for eligibility, of which 32 studies fulfilled the inclusion criteria for full-text review (Fig. 1) [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Study characteristics
Out of the 32 studies, 12 (40.7%) were conducted in the USA [15, 19, 20, 24, 33, 35, 37,38,39,40, 42, 44]; three each in the UK (11.1%) [22, 34, 45] and France [18, 30, 46]; two each in Belgium [31, 36], Greece [27, 28], Australia [23, 41], Ireland [29, 32]; and one each in the Netherlands [21], Spain [43], Turkey [17], Israel [16], Denmark [25], and United Arab of Emirates [26]. Studies were conducted in university-affiliated or academic/teaching hospitals [15, 16, 18, 23, 25, 28,29,30,31, 34, 36, 37, 39, 41, 43, 45, 46], tertiary care non-teaching hospitals [24, 26, 33, 35, 38, 42], primary healthcare centres [22, 43], nursing home [19], private general hospital [27], pharmacist managed anticoagulation clinic [20], central medication registration [21], Poison control system [40], single center [32], and patient safety reporting system [44].
Study quality
The quality of the included studies was variable (Fig. 2). Out of the 32 studies, only two studies (6%) met the eleven CASP-related quality assessment criteria for observational studies, while one study met 10 criteria. The key limitations centred on the lack of justification for the method of sampling and sample size, and exposures characteristics which include drugs and associated comorbidities were often poorly described (Fig. 2).
Study design and data collection
About three quarter of the studies (n = 24) used retrospective routinely collected data [15, 16, 19, 21,22,23,24,25,26, 29, 31,32,33,34,35, 37,38,39,40, 42,43,44,45,46], six used prospective observational design [17, 18, 27, 30, 34, 36], while two studies used mixed study design which were combined with a retrospective chart review, prospective observational data, and clinical trial design [20, 28] (Table 1).
Adopted definitions of medication errors
Although a clear definition of medication error was not provided in most of the included sample studies (n = 30), two studies used established definitions used by North Carolina’s Medication Error Quality Initiative (MEQI), and National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) [19, 37].
Prevalence of errors
Proportion of patients who experienced either prescribing, dispensing, or administration errors across the studies ranged from 5.3 to 37.3%. The variability in the rates of errors was often contributed to the range of DOACs being indicated, types of DOACs agent used in the study settings, and consideration of either certian type of error or range of errors across the different stages of medication use process (Table 1).
Prescribing errors and contributory factors
Eighteen studies reported the number (proportion) of patients amongst the study population where prescribing errors were identified. The overall proportion of patients experiencing prescribing error was found to be 20% (95%CI 15–25%) with a 95% prediction interval (95% PrI) between 4 and 43% (Fig. 3).
Prescribing errors was the most commonly reported error types. The proportion of prescribing errors as a proportion of all error types ranged from 70 to 78%. The pooled estimate of error rate was 78% (95%CI 73–82%; I2 = 0, 95%PrI 68–87%) (Fig. 4).
Prescribing errors detected in the studies included related to over or under dosing [15], missed medication doses [18], and inappropriate prescribing in the context of indication, altered renal function (particularly creatinine clearance), advanced age, altered weight, concomitant medications, and prescribed contraindication based on product label guidelines (Table 1). Studies also reported duplicate therapy from different DOAC agents as one of the common prescribing errors.
A study conducted in a tertiary care community hospital in the USA reported that over a third of patients (n = 92, 35.4%) who received rivaroxaban had an inappropriate dose ordered. Forty one of these patients were considered too low, while 51 were considered too high based on the patient’s renal function at the initiation of rivaroxaban [42]. A UK study showed that 12 (16.4%) of the included patients were prescribed the full dose despite having impaired renal function (CrCl < 50) [33]. In a study conducted in Ireland, an inappropriate dose according to CrCl was found in 11% [29]. The study conducted in a community hospital by Tellor et al. to evaluate the appropriateness of rivaroxaban’s dosing, indication, and safety reported that 36.9% of patients treated for NVAF and 12.4% treated for VTE were on an inappropriate regimen [42]. Furthermore, twenty patients (7.7%) were treated for NVAF with an unapproved 10 mg dose of rivaroxaban, whereas two patients (0.8%) had an inappropriately high dosing frequency of twice daily. Although rivaroxaban is only approved for NVAF, six patients (2.3%) were treated for atrial fibrillation as off label use [42].
Administration error
Only two studies reported the rate of administration errors amongst all types of medication errors associated with DOACs. Heterogeneity was low (I2 = 0), though there were little data to estimate between-study variance. The pooled estimate gave a prevalence of errors to be 5% (95% CI 3–8%). However, nearly all of the weight in the meta-analysis is given to one study [33]. Therefore, there were insufficient studies to calculate a prediction interval (Fig. 5).
A prospective study conducted at a private general hospital in Greece reported that Apixaban was the most frequent DOACs agent to be erroneously administered (13 of 76 cases, 17.1%), followed by rivaroxaban (28 of 257 cases, 10.9%) and dabigatran (one of 37 cases. 2.7%) [27]. One study reported that DOACs were often unavailable on the ward and patients went as long as 48 h without anticoagulation while admitted [45]. Another study reported that 24 patients (23%) were taking rivaroxaban inappropriately without food, and six patients (14%) endorsed inappropriate storage of dabigatran [39]. A study by Stevenson et al. described an administration error related to dabigatran, in which patients mistakenly ingested the drug or were given another patient’s medication [40].
Dispensing errors
The prevalence of dispensing errors amongst all medication errors associated with DOACs was described in one study. This study reports that out of 25 total prescribing errors associated with DOACs, only 2 (8%, 95% CI = 0–18.6%) were identified as dispensing errors [45].
Subgroup analyses
Subgroup analyses of error prevalence across various indications and study settings are provided in electronic supplementary material 3.
Consequences, severity of errors
Only four included studies [15, 25, 40, 44] assessed the severity of harm associated with the error. Haemorrhage was the most frequently reported adverse event associated with the errors especially in patients with lower CrCl and older age. For instance in a US study, haemorrhage occurred in 70.2% of the included subjects and of whom almost 40% of the patients were 80 years or older; furthermore, mortality reported in two patients in this study [44]. However detailed causality assessment linking errors with adverse events were missing. In another study in Danish patients prescribed dabigatran, two incidents of potentially fatal outcomes were also reported [25].
Contributory factors
Of the 32 studies, only 27 reported contributory factors associated with medication errors related to DOACs, yet none of these 27 studies used any theory (e.g., behavioural or organisational) in their methodology or data collection or analysis. The results from these studies were categorised according to Reason’s accident causation model and showed in Table 1. The most commonly reported contributing factors were active failures and mistakes, which included failure to consider risk factors of creatinine clearance, age, or weight when prescribing such medicines. In addition to the inexperience and lack of knowledge of the prescriber, lack of inter-professional collaboration and poor communications with other healthcare providers or with the patients, as well as staffing and workload issues, were also reported as contributory factors that lead to medication errors associated with DOAC (Table 1).
Discussion
Oral anticoagulants are currently among the most widely prescribed medications in clinical practice, with DOACs becoming more and more utilized over VKAs. DOACs have more predictable pharmacokinetic profiles, lower bleeding risks, and fewer drug interactions than warfarin [47]. Complexity of patients’ conditions and polypharmacy therapeutics on the other hand can increase the risk of adverse events. The popularity of DOACs and their associated adverse events made them of interest to investigate errors and identify their contributing factors. The aim of this study was to systematically review the prevalence of medication errors associated with DOACs and its contributory factors. Studies reported a wide prevalence rates often influenced by the method used for error definition and detection, drugs being investigated for errors, and the patient population.
Prevalence of errors
This systematic review found that prescribing errors, particularly the dose-related, were the most prevalent type of errors associated with DOACs. Although DOACs are given in fixed doses and not requiring routine coagulation monitoring, dosing varies based on drug used, indication, renal function, age, and body weight, as well as concurrent medications [48,49,50,51,52]. The nature of errors identified in the systematic review was reflective of these factors.
Severity of errors
The severity of medication incident reports may help identify possible areas for improvement in reporting the adverse events and types of errors related to DOACs. Few studies had reported the severity of adverse events associated with medication errors. New thrombotic and bleeding events risks are found associated with suboptimal prescribing [53], while the likelihood of hospitalisation and mortality increases with overdosing. Detailed causality assessments were however often missing in the included studies as to whether the errors primarily contributed to the adverse events.
Implications for practice
There are several risk reduction strategies relevant to minimise and avoid harm related to medications errors with DOACs. Development of novel-theory based and technology-enabled interventions can improve patient safety. Education of healthcare professionals through training sessions and adopting anticoagulant stewardship programme can be effective. Secondly, undertaking medication reconciliation on admission and discharge as well as upon care transfer in combination with medication reviews.
The relative ease of prescribing and monitoring DOACs compared to VKAs makes them first prescribing choice for many indications like a non-valvular atrial fibrillation and VTE. Such prescribing preference could lead to increased incidence of different types of prescribing errors. Each DOAC has a different dosing schedule and dose adaptations, mostly reductions, depending on one or more patient-specific factors including age, weight, renal function, indication, and concomitant medications. As seen in the included studies, one of the reasons for overdosing DOACs was failing to adjust the dose of DOACs for specific indication, renal function, age, and/or weight. Similarly, elderly patients who are at high risk of developing stroke are often likely to be underdosed. Amongst the included studies which reported nearly fatal or fatal adverse events linked to prescribing errors, severe incidents most commonly occurred during sector change such as admissions, discharge, or undergoing surgery [25, 44]. It is imperative that additional precautions be applied during patient transitions across sectors as well as prior to and after surgical procedures.
This review has shown that the contributing factors to medication errors with DOACs are multifactorial. These factors include inappropriate drug selection and lack of dose adjustments consideration due to failure to approach patients holistically by assessing, for instance, renal function, medical, or medication histories or demographics. Inexperience, poor communication, and lack of inter-professional collaborative practice together with non-compliance to clinical guidelines also contributed to their inappropriate prescribing. Therefore, adopting inter-professional team-based clinical practice and leaning as well as pharmacist-led anticoagulant stewardship program are likely to minimise errors. A recent meta-analysis showed that including a pharmacist in clinical rounds alongside educational interventions and prescription reviews can significantly reduce prescribing errors by as much as three quarters [54].
Implications for research
Future studies should expand on the current research to determine techniques to reduce the occurrence of the more prevalent errors associated with DOACs. Behavioural frameworks such as the theoretical domains framework (TDF) are useful in identifying target behaviours and future interventions [55]. Future studies should consider data from non-hospital settings and undertake rigorous causality assessment to investigate the link between errors and adverse outcomes.
Study strengths and limitations
To the best of our knowledge, this is the first systematic review and meta-analysis that aim to review the prevalence of DOACs associated errors and its contributed factors. A wide range of databases was used including grey literature. However, the current review was limited to the literature published in English language. Overall, the review endorsed the variability of clinical implications and consequences of errors based on patients’ characteristics such as age, co-morbidities, and concomitant of drug therapy.
Furthermore, this review used Reason’s accident causation model to analyse the data related to the error causation. This model focuses on the system or the environment in which the error occurred, rather than the individual that caused the error, and the random rather than intentional act. However, it is important to note that classifying errors based on this model could be subject to the researchers’ interpretation bias, particularly when the conditions of the error were not thoroughly described. Therefore, mutually exclusive classification of the documented medication errors is not always possible. In addition, well-known causes of medication errors under-reporting such as perceived fears of blame, punishment, or indemnity either by patients, clinicians or administration, consequences of reporting protocol, heavy workload, and lack of time will impede estimating the true prevalence of actual errors reported in the included studies [56]. Therefore, studies that rely on incident reporting databases to identify error rates are likely to be provide underestimation of true prevalence [57].
Conclusions
This systematic review and meta-analysis suggests that despite their favourable safety profile and relative ease of use compared to VKAs, medication errors with DOACs are common. Future studies should consider data from non-hospital settings and undertake rigorous causality assessment to investigate the link between errors and adverse outcomes. There is a need to promote multidisciplinary working, guideline-adherence, training and education of healthcare professionals, and the use of theory-based and technology facilitated interventions to minimise errors and maximise the benefits of DOAC usage in all settings.
Data Availability
All data generated or analysed during this study are included in this manuscript.
References
National institute for Health and Care Excellence (2014) Atrial fibrillation: management Available at: https://www.nice.org.uk/guidance/cg180. Accessed 10 July 2021
Joint Formulary Committee (2020) British National Formulary 79. Available at: http://www.medicinescomplete.com. Accessed: 10 July 2021
Hellenbart EL, Faulkenberg KD, Finks SW (2017) Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc Health Risk Manag 13:325–342
Raschi E, Bianchin M, Ageno W, De Ponti R, De Ponti F (2016) Risk-benefit profile of direct-acting oral anticoagulants in established therapeutic indications: an overview of systematic reviews and observational studies. Drug Saf 39(12):1175–1187
Steiner T, Böhm M, Dichgans M, Diener HC, Ell C, Endres M, Epple C, Grond M, Laufs U, Nickenig G, Riess H, l (2013) Recommendations for the emergency management of complications associated with the new direct oral anticoagulants (DOACs), apixaban, dabigatran and rivaroxaban. Clin Res Cardiol 102(6):399–412
Barr D, Epps QJ (2019) Direct oral anticoagulants: a review of common medication errors. J Thromb Thrombolysis 47(1):146–154
Archer S, Hull L, Soukup T, Mayer E, Athanasiou T, Sevdalis N, Darzi A (2017) Development of a theoretical framework of factors affecting patient safety incident reporting: a theoretical review of the literature. BMJ Open 7(12):e017155
Reason J (2000) Human error: models and management. BMJ 320(7237):768–770
Thomas B, Paudyal V, MacLure K, Pallivalapila A, McLay J, El Kassem W, Al Hail M, Stewart D (2019) Medication errors in hospitals in the Middle East: a systematic review of prevalence, nature, severity and contributory factors. Eur J Clin Pharmacol 75(9):1269–1282
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1
Johnson N, Phillip M (2018) Rayyan for systematic reviews. J Elect Res Lib 30(1):46–48
CASP UK (2021) Critical appraisal skills programme. Available: https://casp-uk.net/casp-tools-checklists/. Accessed 21 May 2021
Higgins JP, Thompson SG, Spiegelhalter DJ (2009) Re-evaluation of random-effects meta-analysis. J Royal Stat Soc 172(1):137–159
Reason J (2017) A life in error: from little slips to big disasters. Ashgate Publishing Ltd., Farnham
Alghadeer S, Hornsby L (2017) Assessment of novel oral anticoagulant use within a community teaching hospital. Saudi Pharm J 25(1):93–98
Angel Y, Zeltser D, Berliner S, Ingbir M, Shapira I, Shenhar-Tsarfaty S, Rogowski O (2019) Hospitalization as an opportunity to correct errors in anticoagulant treatment in patients with atrial fibrillation. Brit J Clin Pharmacol 85(12):2838–2847
Basaran O, Filiz Basaran N, Cekic EG, Altun I, Dogan V, Mert GO, Mert KU, Akin F, Soylu MO, Memic Sancar K, Biteker M (2017) Prescription patterns of oral anticoagulants in nonvalvular atrial fibrillation (PROPER study). Clin Appl Thromb-Hem 23(4):384–391
Bruneau A, Schwab C, Anfosso M, Fernandez C, Hindlet P (2019) Burden of inappropriate prescription of direct oral anticoagulants at hospital admission and discharge in the elderly: a prospective observational multicenter study. Drugs Aging 36(11):1047–1055
Desai RJ, Williams CE, Greene SB, Pierson S, Caprio AJ, Hansen RA (2013) Exploratory evaluation of medication classes most commonly involved in nursing home errors. J Am Med Dir Assoc 14(6):403–408
Donaldson M, Norbeck AO (2013) Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract 11(2):90
Dreijer AR, Diepstraten J, Bukkems VE, Mol PG, Leebeek FW, Kruip MJ, van den Bemt PM (2019) Anticoagulant medication errors in hospitals and primary care: a cross-sectional study. Int J Qual Health C 31(5):346–352
Ghai A, Duffus I (2017) Audit of new oral anticoagulant monitoring in primary care: are patients being prescribed the correct dose? Heart 103(5):A37–A37
Glendinning D et al (2016) Prescribing errors with new oral anticoagulants at a regional base hospital in NSW, Australia: CA50. J Thromb Haemost 14:29–30
Greenberg-Schwartz B et al (2015) Decreasing Rivaroxaban medication incidents by pharmacy staff education of the" LEARN" acronym at a community hospital. J Thromb Thrombolysis Drug Res 30:3311
Henriksen J, Nielsen L, Hellebek A et al (2017) Medication errors involving anticoagulants: data from the Danish patient safety database. Pharmacol Res Perspect 5(3):e00307
Hussain S, Gebran N, Hussain K et al (2013) Drug use evaluation of dabigatran in a tertiary care hospital in United Arab Emirates. Eur J Hosp Pharm-S P 20(2):106–109
Loannidis K, carlatinis I, Papachristos et al (2018) Misuse of novel oral anticoagulants in hospital settings. Eur J Hospital Pharm (25):S1:A49
Kartas A, Samaras A, Vasdeki D et al (2019) Flaws in anticoagulation strategies in patients with atrial fibrillation at hospital discharge. J Cardiovasc Pharm T 24(3):225–232
Keohane S, Sandys V, Barry M et al (2016) NOACs: are we prescribing appropriately? Pharmacoepidem Drug Saf 07030–5774
Lafon T et al (2018) Misuse and adverse effects of new direct oral anticoagulants: a prospective observational study in patients admitted to an emergency unit of a French university hospital. Therapie 73(3):209–215
Moudallel S, Steurbaut S, Cornu P, Dupont A (2018) Appropriateness of DOAC prescribing before and during hospital admission and analysis of determinants for inappropriate prescribing. Front Pharmacol 9:1220
Pharithi RB, Ranganathan D, O’Brien J, Egom EE, Burke C, Ryan D, McAuliffe C, Vaughan M, Coughlan T, Morrissey E, McHugh J (2019) Is the prescription right? A review of non-vitamin K antagonist anticoagulant (NOAC) prescriptions in patients with non-valvular atrial fibrillation. Safe prescribing in atrial fibrillation and evaluation of non-vitamin K oral anticoagulants in stroke prevention (SAFE-NOACS) group. Irish J Med Sci 188(1):101–108
Piazza G, Nguyen TN, Cios D, Labreche M, Hohlfelder B, Fanikos J, Fiumara K, Goldhaber SZ (2011) Anticoagulation-associated adverse drug events. Am J Med 124(12):1136–1142
Roberts DM, Burnet IL, Jawad Ul Qamar M (2021) Use of ESC guidelines to influence safe prescribing of oral anticoagulants in AF in relation to renal function. Eur Heart J 38(suppl_1):ehx504.P3611
Schwartz B (2013) Dabigatran monitoring using the acronym ‘Pharmacist’s CARE’ about Dabigatran (Pradaxa (R)) to improve patient outcomes in a community hospital. J Thromb Thrombolysis 30:3311
Sennesael AL, Larock AS, Devalet B, Mathieux V, Verschuren F, Muschart X, Dalleur O, Dogné JM, Spinewine A (2018) Preventability of serious thromboembolic and bleeding events related to the use of oral anticoagulants: a prospective study. Brit J Clin Pharmaco 84(7):1544–1556
Sharma M, Krishnamurthy M, Snyder R, Mauro J (2017) Reducing error in anticoagulant dosing via multidisciplinary team rounding at point of care. Clin Pract 7(2):953
Sheikh-Taha M, Deeb ME (2019) Assessment of non-vitamin K oral anticoagulants use in a tertiary care center in the USA: a chart review of 909 Patients. Am J Cardiovasc Drugs 19(2):195–201
Simon J, Hawes E, Deyo Z, Bryant SB (2015) Evaluation of prescribing and patient use of target-specific oral anticoagulants in the outpatient setting. J Clin Pharm Ther 40(5):525–530
Stevenson JW, Minns AB, Smollin C, Albertson TE, Cantrell FL, Tomaszewski C, Clark RF (2014) An observational case series of dabigatran and rivaroxaban exposures reported to a poison control system. Am J Emerg Med 32(9):1077–1084
Suknate A, Lim YM, Chua GS, Anpalahan M et al (2018) Direct oral anticoagulants: prescription errors and adverse outcomes. Intern Med J 48:9–22
Tellor KB, Patel S, Armbruster AL, Daly MW (2015) Evaluation of the appropriateness of dosing, indication and safety of rivaroxaban in a community hospital. J Clin Pharm Ther 40(4):447–451
Troncoso A, Diogène E (2014) Dabigatran and rivaroxaban prescription for atrial fibrillation in Catalonia, Spain: the need to manage the introduction of new drugs. Eur J clin pharmacol 70(2):249
Valentine D, Gaunt MJ, Grissinger M (2018) Identifying patient harm from direct oral anticoagulants. Pa Patient Saf Advis 15(2). http://patientsafety.pa.gov/ADVISORIES/Pages/201806_DOACs.aspx. Accessed 21 June 2021
Ware V, Petrie C, Talks K (2016) DOACs: Common and uncommon errors. A brief patient safety intervention and review: https://www.postersessiononline.eu/173580348_eu/congresos/BSH2016/aula/-P_168_BSH2016.pdf. Accessed 10 July 2021
Viprey M, Jeannin R, Piriou V, Chevalier P, Michel C, Aulagner G, Berthiller J, Armoiry X (2017) Prevalence of drug-related problems associated with direct oral anticoagulants in hospitalized patients: a multicenter, cross-sectional study. J Clin Pharm Ther 42(1):58–63
Almarshad F, Alaklabi A, Bakhsh E, Pathan A, Almegren M (2018) Use of direct oral anticoagulants in daily practice. Am J Blood Res 8(4):57–72
Ageno W, Crowther M, Baglin T, Falanga A, Buller H, Palareti G (2013) Selection and assessment of patients treated with the novel oral anticoagulant drugs: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 11(1):177–179
European Medicines Agency (undated) Eliquis [apixaban], product information. Available from https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf. Accessed 21 July 2021
European Medicines Agency (undated) Lixiana [edoxaban], product information. Available from https://www.ema.europa.eu/en/documents/product-information/lixiana-epar-product-information_en.pdf. Accessed 10 July 2021
EMA and Pradaxa (undated) European Medicines Agency. Pradaxa [dabigatran etexilate], product information. Available from https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_en.pdf. Accessed 21 June 2021
EMA and Xarelto (undated) European Medicines Agency. Xarelto [rivaroxaban], product information.Available from https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_en.pdf. Accessed 21 June 2021
Howard M, Lipshutz A, Roess B, Hawes E, Deyo Z, Burkhart JI, Moll S, Shilliday BB (2017) Identification of risk factors for inappropriate and suboptimal initiation of direct oral anticoagulants. J Thromb Thrombolys 43(2):149–156
Naseralallah LM, Hussain TA, Jaam M, Pawluk SA (2020). Impact of pharmacist interventions on medication errors in hospitalized pediatric patients: a systematic review and meta-analysis. Int J Clin pharm pp 1–16
Atkins L et al (2017) A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci 12(1):77
Thomas B et al (2019) Medication errors in hospitals in the Middle East: a systematic review of prevalence, nature, severity and contributory factors. Eur J Clin pharmacol 75(9):1269–1282
Haque H, Alrowily A, Jalal Z, Tailor B, Efue V, Sarwar A, Paudyal V (2021) Direct oral anticoagulant-related medication incidents and pharmacists’ interventions in hospital in-patients: evaluation using reason’s accident causation theory. Int J Clin Pharm. https://doi.org/10.1007/s11096-021-01302-6
Acknowledgements
We would like to thank the University of Birmingham Library services for their support in the search process.
Funding
AA was sponsored by the Saudi Ministry of Education for the PhD programme. No other funding was received for the conduct of this study. MJP was supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham.
Author information
Authors and Affiliations
Contributions
This study relates to AA’s PhD research. VP and ZJ supervised AA for his PhD. All authors co-designed the study. Literature search performed by AA and verified by MA and MHA. Literature retrieving, screening, summarising, data extraction, and quality assessment were undertaken by AA, MHA, MA, and HA. Meta-analysis was conducted by MP and AA and reviewed by VP, ZJ, and MHA. The first manuscript draft was written by AA. All authors reviewed and approved for submission.
Corresponding author
Ethics declarations
Ethics statement
Not applicable.
Conflict of interest
The authors declare no competing interests.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Rowily, A., Jalal, Z., Price, M.J. et al. Prevalence, contributory factors and severity of medication errors associated with direct-acting oral anticoagulants in adult patients: a systematic review and meta-analysis. Eur J Clin Pharmacol 78, 623–645 (2022). https://doi.org/10.1007/s00228-021-03212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03212-y