Abstract
Purpose
The purpose of this study was to analyse the effects of demographic factors, clinical factors, and genetic polymorphisms of related gene loci on warfarin bleeding-related complications in the Han population.
Methods
Retrospective medical record review. The study cases were patients treated at the Fujian Medical University Union Hospital from March 2016 to February 2020, and all received regular warfarin anticoagulation treatment for at least 3 months, and were provided the initial standard dose and stable dose of warfarin.
Results
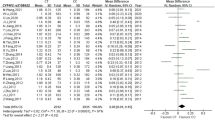
Data were collected from 451 qualifying patients (47% male, 53% female). The average age of patients was 53.8 ± 12.2 years, and the average body surface area was 1.6 ± 0.18 m2. There were nine major bleeding events and 141 minor bleeding events. In the univariate logistic analysis, the p-value of the four factors body weight, body surface area (BSA), amiodarone, and rs429358 was < 0.10. However, the final p-values for amiodarone and rs429358 were < 0.05 in the multifactorial logistic analysis.
Conclusions
The ApoE (rs429358) gene polymorphism influences bleeding complications in Chinese Han patients treated with warfarin. The sample size of this study was relatively small; hence an international study with a larger sample size is needed in the future.

Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Code availability
Not applicable.
References
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW (2019) 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 140(2):e125–e151. https://doi.org/10.1161/CIR.0000000000000665
Crader MF, Johns T, Arnold JK (2020) Warfarin Drug Interactions. StatPearls
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L (2016) Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest 149(2):315–352. https://doi.org/10.1016/j.chest.2015.11.026
Hering D, Piper C, Bergemann R, Hillenbach C, Dahm M, Huth C, Horstkotte D (2005) Thromboembolic and bleeding complications following St. Jude Medical valve replacement: results of the German Experience With Low-Intensity Anticoagulation Study. Chest 127:53–59. https://doi.org/10.1378/chest.127.1.53
Akhtar RP, Abid AR, Zafar H, Khan JS (2009) Anticoagulation in patients following prosthetic heart valve replacement. Ann Thorac Cardiovasc Surg 15:10–17. https://doi.org/10.1177/021849230701500606
Taube J, Halsall D, Baglin T (2000) Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over anticoagulation in patients on long-term treatment. Blood 96(5):1816–1819. https://doi.org/10.1182/blood.V96.5.1816.h8001816_1816_1819
Schelleman H, Chen Z, Kealey C, Whitehead AS, Christie J, Price M, Brensinger CM, Newcomb CW, Thorn CF, Samaha F, Kimmel SE (2007) Warfarin response and vitamin K epoxide reductase complex 1 in African Americans and Caucasians. Clin Pharmacol Ther 81(5):742–747. https://doi.org/10.1038/sj.clpt.6100144
Meckley LM, Wittkowsky AK, Rieder MJ, Rettie AE, Veenstra DL (2008) An analysis of the relative effects of VKORC1 and CYP2C9 variants on anticoagulation related outcomes in warfarin-treated patients. Thromb Haemost 100(2):229–239. https://doi.org/10.1160/TH07-09-0552
Jorgensen AL, Al-Zubiedi S, Zhang JE, Keniry A, Hanson A, Hughes DA, van Eker D, Stevens L, Hawkins K, Toh CH, Kamali F, Daly AK, Fitzmaurice D, Coffey A, Williamson PR, Park BK, Deloukas P, Pirmohamed M (2009) Genetic and environmental factors determining clinical outcomes and cost of warfarin therapy: A prospective study. Pharmacogenet Genom 19(10):800–812. https://doi.org/10.1097/FPC.0b013e3283317ab5
Molden E, Okkenhaug C, Ekker SE (2010) Increased frequency of CYP2C9 variant alleles and homozygous VKORC1*2B carriers in warfarin-treated patients with excessive INR response. Eur J Clin Pharmacol 66(5):525–530. https://doi.org/10.1007/s00228-010-0813-6
Zhong SL, Yu XY, Liu Y, Xu D, Mai LP, Tan HH, Lin QX, Yang M, Lin SG (2012) Integrating interacting drugs and genetic variations to improve the predictability of warfarin maintenance dose in Chinese patients. Pharmacogenet Genom 22(3):176–182. https://doi.org/10.1097/FPC.0b013e32834f45f9
Wadelius M, Chen LY, Lindh JD, Eriksson N, Deloukas P (2009) The largest prospective warfarin-treated cohort supports genetic forecasting. Blood 113(4):784–792. https://doi.org/10.1182/blood-2008-04-149070
Limdi NA, McGwin G, Goldstein JA, Beasley TM, Arnett DK, Adler BK, Baird MF, Acton RT (2008) Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther 83(2):312–321. https://doi.org/10.1038/sj.clpt.6100290
Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Rettie AE (2002) Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 287(13):1690–1698. https://doi.org/10.1001/jama.287.13.1690
Wu KK, Aleksic N, Ahn C, Boerwinkle E, Folsom AR, Juneja H (2001) Thrombomodulin Ala455Val polymorphism and risk of coronary heart disease. Circulation 103(10):1386–1389. https://doi.org/10.1161/01.CIR.103.10.1386
Ireland H, Kunz G, Kyriakoulis K, Stubbs PJ, Lane DA (1997) Thrombomodulin gene mutations associated with myocardial infarction. Circulation 96(1):15–18. https://doi.org/10.1161/01.CIR.96.1.15
Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A (2005) Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics 6(2):115–138. https://doi.org/10.1517/14622416.6.2.115
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M (2000) Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 97(7):3473–3478. https://doi.org/10.1073/pnas.050585397
Morita Y, Sakaeda T, Horinouchi M, Nakamura T, Kuroda K, Miki I, Yoshimura K, Sakai T, Shirasaka D, Tamura T, Aoyama N, Kasuga M, Okumura K (2003) MDR1Genotype-Related Duodenal Absorption Rate of Digoxin in Healthy Japanese Subjects. Pharm Res (Dordrecht) 20(4):552–556. https://doi.org/10.1023/A:1023282312757
Tavares LC, Marcatto LR, Soares RAG, Krieger JE, Pereira AC, Santos PCJL (2018) Association Between ABCB1 Polymorphism and Stable Warfarin Dose Requirements in Brazilian Patients. Front Pharmacol 9:542. https://doi.org/10.3389/fphar.2018.00542
De Oliveira Almeida VC, De Souza Ferreira AC, Ribeiro D, Gomes Borges KB, Salles Moura Fernandes AP, Brunialti Godard AL (2011) Association of the C3435T polymorphism of the MDR1 gene and therapeutic doses of warfarin in thrombophilic patients. J Thromb Haemostasis 9(10):2120–2122. https://doi.org/10.3389/fphar.2018.00542
Li W, Zhao P, Chen L, Lai X, Shi G, Li L, Dong J (2020) Impact of CYP2C9, VKORC1, ApoE and ABCB1 Polymorphisms on Stable Warfarin Dose Requirements in Elderly Chinese Patients. Pharmacogenomics 21(2):101–110. https://doi.org/10.2217/pgs-2019-0139
Gage BF, Eby CS (2004) The genetics of vitamin K antagonists. Pharmacogenomics J 4(4):224–225. https://doi.org/10.1038/sj.tpj.6500258
Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, Wallerman O, Melhus H, Wadelius C, Bentley D (2005) Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J 5(4):262–270. https://doi.org/10.1038/sj.tpj.6500313
Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, Bentley D, McGinnis R, Deloukas P (2007) Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 121(1):23–34. https://doi.org/10.1007/s00439-006-0260-8
Li B, Liu R, Wang C, Ren C, Zhang S, Zhang F, Zhang J, Wei SLY, Liu W, Song B, Xinan Wu (2019) Impact of Genetic and Clinical Factors on Warfarin Therapy in Patients Early After Heart Valve Replacement Surgery. Eur J Clin Pharmacol 75(12):1685–1693. https://doi.org/10.1007/s00228-019-02747-5
Kishino S, Nomura A, Di ZS, Sugawara M, Iseki K, Kakinoki S, Kitabatake A, Miyazaki K (1995) Alpha-1-acid glycoprotein concentration and the protein binding of disopyramide in healthy subjects. J Clin Pharmacol 35(5):510–514. https://doi.org/10.1002/j.1552-4604.1995.tb04096.x
Eap CB, Cuendet C, Baumann P (1988) Binding of amitriptyline to alpha 1-acid glycoprotein and its variants. J Pharm Pharmacol 40(11):767–770. https://doi.org/10.1111/j.2042-7158.1988.tb05169.x
Wang LS, Shang J, Shi SY, Zhang YQ, Lin J, Guo ZH, Wang YC, Tang J, Liu J, Liu YZ, Li Z, Tan ZR, Zhou HH, Jiang HH, Xie HT (2013) Influence of ORM1 polymorphisms on the maintenance stable warfarin dosage. Eur J Clin Pharmacol 69(5):1113–1120. https://doi.org/10.1007/s00228-012-1448-6
Timson DJ (2017) Dicoumarol: A Drug which Hits at Least Two Very Different Targets in Vitamin K Metabolism. Curr Drug Targets 18(5):500–510. https://doi.org/10.2174/1389450116666150722141906
Li J, Yang W, Xie Z, Kun Yu, Chen Y, Cui K (2018) Impact of VKORC1, CYP4F2 and NQO1 Gene Variants on Warfarin Dose Requirement in Han Chinese Patients With Catheter Ablation for Atrial Fibrillation. BMC Cardiovasc Disord 18(1):96. https://doi.org/10.1186/s12872-018-0837-x
Wennberg P, Wensley F, Di Angelantonio E, Johansson L, Boman K, Rumley A, Lowe G, Hallmans G, Danesh J, Jansson JH (2012) Haemostatic and inflammatory markers are independently associated with myocardial infarction in men and women. Thromb Res Jan 129(1):68–73. https://doi.org/10.1016/j.thromres.2011.05.015
Vene N, Mavri A, Kosmelj K, Stegnar M (2003) High d-dimer levels predict cardiovascularevents in patients with chronic atrial fibrillation during oral anticoagulant therapy. Thromb Haemost 90(6):1163–1172. https://doi.org/10.1160/TH03-06-0363
Lind M, Boman K, Johansson L, Nilsson TK, Järvholm LS, Jansson JH (2014) D-dimer predicts major bleeding, cardiovascular events and all-cause mortality during warfarin treatment. Clin Biochem 47(7–8):570–573. https://doi.org/10.1016/j.clinbiochem.2014.03.003
Abdi AA, Osman A (2017) Prevalence of common hereditary risk factors for thrombophilia in Somalia and identification of a novel Gln544Arg mutation in coagulation factor V. J Thromb Thrombolysis 44(4):536–543. https://doi.org/10.1007/s11239-017-1543-8
Chen M, Mao BY, Wang D, Cheng X, Xu CX (2016) Association between rs1801133 polymorphism and risk of adult ischemic stroke: Meta-analysis based on case–control studies. Thromb Res 137:17–25. https://doi.org/10.1016/j.thromres.2015.11.037
Ezigbo ED, Ukaejiofo EO, Nwagha TU (2016) Molecular characterization of exon 28 of von Willebrand′s factor gene in Nigerian population. Niger J Clin Pract 20(2):235–238. https://doi.org/10.4103/1119-3077.197002
Ma C, Zhang Y, Qiang Xu, Yang J, Zhang Y, Gao L, Bin Xu, Wang H, Li Y, Caiyi Lu, Yin T (2012) Influence of warfarin dose-associated genotypes on the risk of hemorrhagic complications in Chinese patients on warfarin. Int J Hematol 96(6):719–728. https://doi.org/10.1007/s12185-012-1205-8
Tavares LC, Duarte NE, Marcatto LR, Soares RA, Krieger JE, Pereira AC, Santos PC, Lima J (2018) Impact of incorporating ABCB1 and CYP4F2 polymorphisms in a pharmacogenetics-guided warfarin dosing algorithm for the Brazilian population. Eur J Clin Pharmacol 74(12):1555–1566. https://doi.org/10.1007/s00228-018-2528-z
Bai Y, Chen J, Sun K, Wang Y, Hui R (2012) A Functional Variant in Promoter Region of Platelet-Derived Growth factor-D Is Probably Associated with Intracerebral Hemorrhage. J Neuroinflammation 9:26. https://doi.org/10.1186/1742-2094-9-26
Bergrem A, Dahm AE, Jacobsen AF, Mowinckel MC, Sandvik L, Sandset PM (2011) Resistance to activated protein C is a risk factor for pregnancy-related venous thrombosis in the absence of the F5 rs6025 (factor V Leiden) polymorphism. Br J Haematol 154(2):241–247. https://doi.org/10.1111/j.1365-2141.2011.08712.x
Kimmel SE, Christie J, Kealey C, Chen Z, Price M, Thorn CF, Brensinger CM, Newcomb CW, Whitehead AS (2008) Apolipoprotein E genotype and warfarin dosing among Caucasians and African Americans. Pharmacogenomics J 8(1):53–60. https://doi.org/10.1038/sj.tpj.6500445
Liu R, Zhang K, Gong ZZ, Shi XM, Zhang Q, Pan XD, Dong R (2016) Association of apolipoprotein E (APOE) polymorphisms with warfarin maintenance dose in a northern Han Chinese population. Lipids Health Dis 15(1):34. https://doi.org/10.1186/s12944-016-0205-8
He S, Zhang H, Cao Y, Nian F, Chen H, Chen W, Auchoybur ML, Yin L, Tao Z, Tang S, Chen X (2018) Association between apolipoprotein E genotype and warfarin response during initial anticoagulation. Biomed Pharmacother 101:251–256. https://doi.org/10.1016/j.biopha.2018.02.095
Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de Faire U, Danesh J (2007) Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 298(11):1300–1311. https://doi.org/10.1001/jama.298.11.1300
Kimmel SE, Christie J, Kealey C, Chen Z, Price M, Thorn CF, Brensinger CM, Newcomb CW, Whitehead AS (2007) Apolipoprotein E genotype and warfarin dosing among caucasians and African Americans. Pharmacogenom J 8(1):53–60. https://doi.org/10.1038/sj.tpj.6500445
Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, Bentley D, Mcginnis R, Deloukas P (2007) Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 121(1):23–34. https://doi.org/10.1007/s00439-006-0260-8
Funding
This work was supported by the Natural Science Foundation of Fujian Province of China [2018Y0037].
Author information
Authors and Affiliations
Contributions
JZ and XX drafted the manuscript; JZ and XX participated in the design of the study; JZ, XX, JF, TW, WC, SJ, and ML coordinated the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of Union Hospital affiliated to Fujian Medical University (2016KY036).
Consent to participate
All selected patients signed an informed consent form.
Consent for publication
All authors agree to publish.
Conflict of interest
None of the authors has a conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xia, X., Fu, J., Wu, T. et al. Effect of gene polymorphism on bleeding complications in Chinese Han patients taking warfarin. Eur J Clin Pharmacol 78, 205–214 (2022). https://doi.org/10.1007/s00228-021-03204-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03204-y