Abstract
Purpose
Data on the anti-Xa efficacy of fondaparinux in dialysis-dependent chronic kidney disease (DD-CKD) patients are scarce. This study characterizes the pharmacokinetics (PK) and pharmacodynamics (PD) of fondaparinux in DD-CKD patients undergoing renal replacement therapy (RRT), to assess dosing strategies.
Methods
A retrospective, observational study was conducted using data on anti-Xa activity (112 samples) from 12 (3 male and 9 female) DD-CKD patients (median (IQR) age 71 years (63–88), weight 73 kg (59–98.5)). Eleven patients underwent high-flux or low-flux hemodialysis (HD) and one patient underwent peritoneal dialysis. Three patients were also treated with therapeutic plasma exchange (TPE). A non-linear mixed effects analysis was performed using NONMEM 7.3.0.
Results
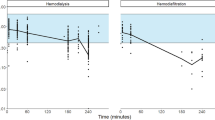
The lab-specific slope of the relationship between fondaparinux concentration and anti-Xa levels was 1.18 IU/µg. In a one-compartment model, clearance (CL) and volume of distribution (Vd) were 0.05289 L/h and 5.55 L, respectively. High-flux HD was found to increase the CL of fondaparinux 2.26 times. TPE also considerably increased CL, but the fold-change could not be accurately estimated. Low-flux HD and peritoneal dialysis did not impact PK parameters.
Conclusions
Model-based simulations showed that standard dosing (2.5 mg three times weekly before HD) results in a median anti-Xa activity of 0.55 IU/mL and 0.98 IU/mL, pre- and post-low-flux HD, respectively. In patients undergoing high-flux HD, these values are approximately 27% lower. Additional caution is warranted with TPE, as this treatment can reduce anti-Xa activity even further.


Similar content being viewed by others
Code availability
The data are not available in any public repository. The model codes will be made available through the model repository of DDMoRe available through: http://repository.ddmore.foundation/.
Change history
16 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00228-021-03220-y
References
Daugirdas J, Blake P, Ing T (2015) Handbook of dialysis. Wolters Kluwer
Haase M, Bellomo R, Rocktaeschel J, Ziemer S, Kiesewetter H, Morgera S, Neumayer HH (2005) Use of fondaparinux (ARIXTRA) in a dialysis patient with symptomatic heparin-induced thrombocytopaenia type II. Nephrol Dial Transplant 20(2):444–446. https://doi.org/10.1093/ndt/gfh544
Brown P, Jay R, Fox A, Oliver M (2013) Chronic fondaparinux use in a hemodialysis patient with heparin-induced thrombocytopenia type II and extracorporeal circuit thrombosis-a case report and review of the literature. Hemodial Int 17(3):444–449. https://doi.org/10.1111/hdi.12003
Taskapan H, Karahan D, Kuku I, Koc S (2010) Heparin-induced thrombocytopenia as a cause of deep venous thrombosis: effectiveness of fondaparinux in dialysis patients. Turkish Nephrology Dialysis Transplantation 19:55–57. https://doi.org/10.5262/tndt.2010.1001.09
European Medicines Agency (2021) (https://www.ema.europa.eu/en/medicines/human/EPAR/arixtra#product-information-section, accessed 06/23/2021) Arixtra: Summary of Product Characteristics
Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A (2013) Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 28(12):3090–3095. https://doi.org/10.1093/ndt/gft293
Mahieu E, Claes K, Jacquemin M, Evenepoel P, Op De Beek K, Bogaert AM, Kuypers D, Verhamme P, Meijers B (2013) Anticoagulation with fondaparinux for hemodiafiltration in patients with heparin-induced thrombocytopenia: dose-finding study and safety evaluation. Artif Organs 37(5):482–487. https://doi.org/10.1111/aor.12002
Hartinger JM, Svobodová A, Malíková I, Šachl R, Slanař O (2020) Effective use of fondaparinux in patient with unresponsiveness to nadroparin. J Clin Pharm Ther. https://doi.org/10.1111/jcpt.13328
Kalicki RM, Aregger F, Alberio L, Lämmle B, Frey FJ, Uehlinger DE (2007) Use of the pentasaccharide fondaparinux as an anticoagulant during haemodialysis. Thromb Haemost 98(6):1200–1207. https://doi.org/10.1160/th07-07-0444
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed 79(3):241–257. https://doi.org/10.1016/j.cmpb.2005.04.005
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Methods Prog Biomed 75(2):85–94. https://doi.org/10.1016/j.cmpb.2003.11.003
Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD (2011) Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Prog Biomed 101(1):72–79. https://doi.org/10.1016/j.cmpb.2010.04.018
Donat F, Duret JP, Santoni A, Cariou R, Necciari J, Magnani H, de Greef R (2002) The pharmacokinetics of fondaparinux sodium in healthy volunteers. Clin Pharmacokinet 41(Suppl 2):1–9. https://doi.org/10.2165/00003088-200241002-00001
Comets E, Brendel K, Mentré F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Prog Biomed 90(2):154–166
De Cock RF, Piana C, Krekels EH, Danhof M, Allegaert K, Knibbe CA (2011) The role of population PK–PD modelling in paediatric clinical research. Eur J Clin Pharmacol 67(1):5–16
Paolucci F, Claviés MC, Donat F, Necciari J (2002) Fondaparinux sodium mechanism of action: identification of specific binding to purified and human plasma-derived proteins. Clin Pharmacokinet 41(Suppl 2):11–18. https://doi.org/10.2165/00003088-200241002-00002
Cope J, Bushwitz J, An G, Antigua A, Patel A, Zumberg M (2015) Clinical experience with prophylactic fondaparinux in critically ill patients with moderate to severe renal impairment or renal failure requiring renal replacement therapy. Ann Pharmacother 49(3):270–277. https://doi.org/10.1177/1060028014563325
Delavenne X, Zufferey P, Nguyen P, Rosencher N, Samama CM, Bazzoli C, Mismetti P, Laporte S (2012) Pharmacokinetics of fondaparinux 1.5 mg once daily in a real-world cohort of patients with renal impairment undergoing major orthopaedic surgery. Eur J Clin Pharmacol 68 (10):1403–1410. https://doi.org/10.1007/s00228-012-1263-0
Cheng CW, Hendrickson JE, Tormey CA, Sidhu D (2017) Therapeutic plasma exchange and its impact on drug levels: an ACLPS critical review. Am J Clin Pathol 148(3):190–198. https://doi.org/10.1093/ajcp/aqx056
Turpie A (2004) Fondaparinux: a factor Xa inhibitor for antithrombotic therapy. Expert Opin Pharmacother 5:1373–1384. https://doi.org/10.1517/14656566.5.6.1373
Ho G, Leblanc K, Selby R, Richardson R, Hladunewich M, Battistella M (2013) Use of fondaparinux for circuit patency in hemodialysis patients. Am J Kidney Dis 61(3):525–526. https://doi.org/10.1053/j.ajkd.2012.09.015
Sombolos KI, Fragia TK, Gionanlis LC, Veneti PE, Bamichas GI, Fragidis SK, Georgoulis IE, Natse TA (2008) Use of fondaparinux as an anticoagulant during hemodialysis: a preliminary study. Int J Clin Pharmacol Ther 46(4):198–203
Funding
This work was supported by the Charles University Project Progress Q25 and grant No. SVV 260 523.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by J.M.H., D.M, and E.H.J.K.; Data were collected and retreived by J.M.H. Z.H., V.B., B.S., V.P., V.T.and; A.M. E.H.J.K. supervised the data analysisAll authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by ethical comittee of General University Hoaspital in Prague under reference number 114/21 S-IV.
Consent to participate and consent for publication
At admission, the patients signed an informed consent wherein they agree, inter alia, that anonymous data can be used for research and publication of the research results.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michaličková, D., Hartinger, J.M., Hladinová, Z. et al. Population pharmacokinetics-pharmacodynamics of fondaparinux in dialysis-dependent chronic kidney disease patients undergoing chronic renal replacement therapy. Eur J Clin Pharmacol 78, 89–98 (2022). https://doi.org/10.1007/s00228-021-03201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03201-1