Abstract
Purpose
To study if second-generation antipsychotic (S-GA) use during the first trimester of pregnancy is associated with an increased risk of major congenital malformations (MCM).
Methods
A population-based birth cohort study using national register data extracted from the Drugs and Pregnancy database in Finland, years 1996–2017. The sampling frame included 1,273,987 pregnant women. We included singleton pregnancies ending in live or stillbirth or termination of pregnancy due to severe malformation. Pregnancies with exposure to known teratogens were excluded. Women were categorized into three groups: exposed to S-GAs (n = 3478), exposed to first-generation antipsychotics (F-GAs) (n = 1030), and unexposed (no purchases of S-GAs or F-GAs during pregnancy, n = 22,540). We excluded genetic conditions and compared the prevalence of MCMs in S-GA users to the two comparison groups using multiple logistic regression models.
Results
Use of S-GAs during early pregnancy was not associated with an increased risk of overall MCMs compared to unexposed (adjusted odds ratio, OR 0.92; 95% CI 0.72–1.19) or to F-GA users (OR 0.82; 95% CI 0.56–1.20). Of individual S-GAs, olanzapine use was associated with an increased risk of overall MCMs (OR 2.12; 95% CI 1.19–3.76), and specifically, an increased risk of musculoskeletal malformations (OR 3.71; 95% CI 1.35–10.1) when compared to unexposed, while comparisons to F-GA users did not show significant results.
Conclusions
Olanzapine use is associated with an increased risk of major congenital malformations and specifically, musculoskeletal malformations. Use during pregnancy should be restricted to situations where no safer alternatives exist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maternal well-being is important for a successful pregnancy outcome, and psychiatric illness must be treated adequately also during pregnancy. The clinician is often encountered by this dilemma, optimizing between effective maternal drug treatment and fetal safety. While antipsychotics are primarily used for psychotic illnesses, the second-generation antipsychotics (S-GAs) are also used in bipolar disorder for mood stabilization and in unipolar depression together with antidepressants [1]. Further, off-label use includes use in anxiety disorders, obsessive–compulsive disorders (OCD), post-traumatic stress disorder (PTSD) and insomnia [2].
The use of S-GA has been steadily increasing among pregnant women since year 2000; a recent study from ten countries reported that up to 2% of pregnant women use S-GAs, while there are differences across countries [3]. At the same time, the use of first generation antipsychotics (F-GA) has waned [3, 4]. The increasing use of S-GAs may be at least partly explained by the increasing off-label use of quetiapine for insomnia [2, 5].
Previous studies have not observed an association between F-GA or S-GA use and an increased risk of overall congenital malformations [6,7,8]. For the individual S-GAs, most data are available for aripiprazole, olanzapine, quetiapine and risperidone. Of these, only risperidone use has been associated with a small increased risk of congenital malformations and specifically, a marginally increased risk of congenital heart defects [6, 8]. While very few data are available for other organ specific malformations, no specific associations have been reported [9]. However, teratogens typically affect organ differentiation specifically, based on their pharmacodynamic and biological effects; an increased risk of organ specific malformations may therefore remain undetected when malformations are analyzed all together as a group [8, 10].
We evaluated the risk of overall major congenital malformations (MCM) and organ specific MCMs in offspring exposed to S-GAs in early pregnancy using national register data. Comparisons were made to unexposed and those exposed to F-GAs, controlling for maternal illness.
Methods
Data source and study cohort
This is a population-based birth cohort study using national register data extracted from the existing Drugs and Pregnancy database, established by the Finnish Institute for Health and Welfare (THL), the Social Insurance Institution of Finland (Kela), and the Finnish Medicines Agency (FIMEA). This database enables continuous surveillance of drug safety during pregnancy and includes data from the Medical Birth Register, the Abortion Register, the Register of Congenital Malformations, and the Prescription Register, including also the Special Refund Entitlement Register. Data from the different registers have been linked by the personal identification number assigned to all citizens and permanent residents in Finland. Data from births and terminations of pregnancy, and prescription drug purchases have been collected since January 1, 1996, and data in our study extend until December 31, 2017. In the database, beginning of pregnancy has been calculated from the best clinical estimation of gestational age at birth, primarily based on ultrasound. First trimester is defined as extending from the last menstrual period (LMP) until 84 days gestation.
More detailed information on the registers included have been published previously [4] and are also available in the Supplementary material.
Definition of exposed and unexposed cohorts
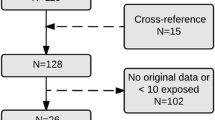
The study frame included 1,273,987 pregnancies ending in livebirth, stillbirth or elective termination of pregnancy due to fetal malformation (Fig. 1). We included 1,235,950 singleton pregnancies. Pregnancies exposed to known teratogens (Supplementary Table S1) during 3 months before pregnancy until end of pregnancy (n = 9876) were excluded from the study (Fig. 1).
Exposed to S-GAs
Women who purchased S-GAs (olanzapine, quetiapine, risperidone, aripiprazole, clozapine, ziprasidone, sertindol, or asenapine; Supplementary Table S2) during 1 month before pregnancy until the end of first trimester (n = 3478).
Unexposed
Women had no S-GA or F-GA purchases during three months before pregnancy until end of first trimester. Controls in this group were matched for year of birth of child and were randomly selected as five controls for one S-GA or F-GA exposed (5:1) (n = 22,540).
Exposed to F-GAs
Women who purchased F-GAs (Supplementary Table S2) during one month before pregnancy until the end of first trimester but did not purchase S-GAs during the same time period. Pregnancies exposed only to prochlorperazine, often used for morning sickness, (n = 392) were excluded. This comparison group was included to control for maternal psychiatric illness (n = 1030).
Major congenital malformations
The outcomes of interest were overall MCMs, i.e., infant/fetus with at least one major congenital malformation and organ-specific malformations, including cardiovascular (ICD-9 diagnoses 745–747), central nervous system (ICD-9; 740–742) respiratory tract malformations (ICD-9; 748), orofacial clefts (ICD-9; 7490–7492), urogenital (ICD-9; 752,753), gastrointestinal malformations (ICD-9; 750,751, 7566), and musculoskeletal malformations (ICD-9; 7543–7548, 755). We excluded genetic conditions from the analyses (Fig. 1).
Covariates
Covariates included maternal sociodemographic and medical characteristics and use of other medication, categorized as shown in Supplementary Table S3. Data on pre-pregnancy BMI are partially available beginning from 2004 and for all women from September 2005. Alcohol use is not routinely collected in the MBR and could therefore not be included in analyses.
Statistical analyses
All data in the Drugs and Pregnancy database are pseudonymized. We made a descriptive analysis on demographic differences between study cohorts. We also calculated the prevalence of overall MCMs and organ-specific malformations in the S-GA group, the unexposed group, and the F-GA group, and further, on the level of individual S-GAs.
We used logistic regression to assess the association between S-GA use during preconception or first trimester and MCMs in comparison with unexposed and F-GA exposed pregnancies. First, pattern of missingness for socioeconomic status, smoking, cohabitation pre-pregnancy BMI, and parity was explored as appropriate. The majority of missingness was driven in one missing value either in socio-economic status or BMI. Incomplete records were assumed to be missing at random, and we used multiple imputation by chained equations (MICEs) to predict missing values in covariates to improve measurement of prognostic factors [11, 12]. Imputation models included outcome variables, exposure variables, covariates and also auxiliary variables that correlated or were believed to be associated with missingness (birth weight, gestational age, number of drug purchases and hospital district). We created 20 imputed data sets of which estimates were combined using Rubin’s rules [13, 14].
The crude models included adjustment for year of delivery. For the adjusted analyses, clinically relevant and plausible covariates were first tested for association with the three-class exposure status. When associated with exposure at significance level P < 0.1, the covariate was further tested separately for association of outcome. We included in the final logistic regression models covariates which were associated with exposure and outcome at P < 0.1 as potential confounders (Supplementary Table S4). An infant/fetus with multiple organ-specific MCMs contributed to each malformation subgroup. All analyses were performed using SAS Enterprise Guide 7.1(SAS Institute Inc., Cary, NC, USA).
The utilization of sensitive health register data for scientific research and the data linkages in the Drugs and Pregnancy project have been approved by the register administrators and the national data protection authority. Since the study subjects are not contacted, according to the Finnish legislation informed consent is not required. The study was registered in The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) register before data collection started (EUPAS4799). The study has been granted the ENCePP seal, following the ENCePP principles of standards, transparency and independence of good pharmacoepidemiology practice throughout the research process (www.encepp.eu).
Results
Overall, 4508 (0.4%) pregnant women with a singleton pregnancy (N = 1,235,950) used antipsychotics during the first trimester or 30 days before pregnancy, and 3478 (0.3%) used S-GAs. The number of stillbirths and ETOPFAs were small across the exposure groups (Fig. 1).
Maternal characteristics of the three study groups are presented in Supplementary Table S3. S-GA users were more likely to be overweight than F-GA users or unexposed and S-GA and F-GA users were more likely to smoke tobacco than women in the unexposed group. Pre-gestational diabetes and gestational diabetes were significantly more common among the S-GA users compared to F-GA users and to the unexposed group.
The numbers of excluded infants/fetuses with genetic conditions are presented in Fig. 1. Of the individual S-GAs, the most commonly used was quetiapine (n = 2618), followed by olanzapine (n = 413), risperidone (n = 242), aripiprazole (n = 220), and clozapine (n = 106).
The prevalence and risk of overall MCMs are presented in Tables 1 and 2. Compared to unexposed, the risk did not differ between any S-GA users and unexposed (Table 1). Of the individual S-GAs, olanzapine use was associated with a twofold increased risk of MCMs (odds ratio, OR 2.12; 95% CI 1.19–3.76) after full adjustment to confounders.
The risk of MCMs in any S-GA users was lower than in the F-GA group; however, this difference was not statistically significant (Table 2). Of the individual S-GAs, olanzapine use was associated with an increased risk of any MCM when compared to F-GA users, but did not reach statistical significance.
Use of any S-GA was not associated with an increased risk of any organ specific malformations when compared to unexposed or to the F-GA group (Tables 3 and 4). Of the individual S-GAs, olanzapine use was associated with a nearly fourfold increased risk of musculoskeletal malformations when compared to unexposed (OR 3.71; 95% CI 1.35–10.1). Compared to F-GA users, the risk of musculoskeletal malformations was higher but the association did not remain statistically significant (Table 4). The musculoskeletal malformations recorded among the olanzapine exposed infants/fetuses were various and no pattern of malformations was observed.
Discussion
In this study based on national register data, use of second-generation antipsychotics during early pregnancy was not associated with an increased risk of overall major congenital malformations compared to unexposed pregnancies or to pregnancies where the woman used first-generation antipsychotics. Of the individual S-GAs, olanzapine use was associated with a twofold increased risk of overall malformations, and specifically, a nearly fourfold increased risk of musculoskeletal malformations when compared to unexposed.
Use of S-GAs has been increasing among the pregnant population [3, 4] and indications for S-GA use have expanded during the last decade to include also illnesses outside psychotic disorders [3, 5]. S-GAs are used increasingly in treating bipolar disorder and as augmentation treatment in unipolar depression [15]. Further, S-GAs may be preferred during pregnancy because of safety concerns related to antiepileptic drugs as mood stabilizers [3]. Antipsychotic augmentation may also be effective in treatment-refractory OCD [16]. Present guidelines state that there is insufficient evidence to recommend for or against antipsychotic medication for PTSD [17] and use should be restricted to situations where symptoms have not responded to other drug or psychological treatments [18]. However, a European study reported that nearly 60% of more than 1,000 patients with a diagnosis of PTSD were prescribed antipsychotics, most commonly quetiapine, olanzapine and risperidone [19]. Further, a population-based study from Norway suggested that the majority of quetiapine prescriptions during 2004–2015 were for indications other than psychosis [5]. S-GAs and particularly quetiapine are used off-label for anxiety disorders and sleep disturbances with little evidence to support use for insomnia [20, 21]. There are no data about S-GA use during pregnancy and specific indications.
Our finding that S-GAs as a group do not increase the risk of congenital malformations is in line with most previous studies. A prospective follow-up study including more than 500 pregnancies following first trimester exposure to S-GAs and including also pregnancy terminations reported an increased risk of overall malformations in S-GA users when compared to unexposed (OR, 2.13; 95% CI, 1.19–3.83) but the risk was mainly attributed to cardiac septal defects, and the authors concluded that the results likely resulted from detection bias [9]. No increased risk of congenital malformations was observed in a cohort study based on health administrative databases in Canada and including approximately 1,000 S-GA exposed pregnancies ending in birth, applying high dimensional propensity score matching analyses [7]. Also, the largest study based on Medicaid data including more than 9,000 pregnancies exposed to S-GAs and ending in live birth did not observe an increased risk of overall (OR 1.05; 95% CI 0.96–1.16) or cardiac (OR 1.06; 95% CI 0.90–1.24) malformations in the fully adjusted, propensity score-based analysis. Of the individual drugs, only risperidone use (> 1500 pregnancies) was associated with an increased risk of overall malformations (OR 1.26; 95% CI 1.02–1.56) and a marginally significant risk of cardiac malformations (OR 1.26; 95% CI 0.88–1.81) [6]. No sign of teratogenicity has been reported for aripiprazole, quetiapine, or olanzapine; the number of reported olanzapine exposed pregnancies analyzed for malformations totals close to 3,000 pregnancies [6, 8, 22]. Contrary to these findings, olanzapine use in our study was associated with a substantially increased risk of overall malformations and specifically with musculoskeletal malformations when compared to the unexposed cohort.
The association of olanzapine use with an increased risk of overall malformations was unexpected, given the reassuring data from the previous studies [6,7,8]. Very few data exist for other specific malformations than congenital cardiac defects. It is possible that an increased risk of organ specific malformations (other than cardiac defects) may have remained undetected in studies which have focused on the overall malformation risk. Olanzapine has not been teratogenic in animal studies.
One study based on prospectively collected data in relation to outcome reported no major differences in organ specific malformations, including musculoskeletal malformations in S-GA users compared to F-GA users or to pregnancies with no exposure but the total number of S-GA exposed pregnancies available for analyses was only 430, and no results were reported for individual S-GAs [9]. S-GA treatment may cause disturbances in glucose metabolism, leading to hyperglycemia and diabetes, and poor glycemic control before pregnancy and in early pregnancy is associated with an increased risk of congenital malformations [23, 24]. Typical malformations related to maternal hyperglycemia include cardiovascular and central nervous system malformations, but also musculoskeletal malformations [23, 25]. While impaired glucose metabolism is a common side effect of all S-GAs, one would expect to find an increased malformation risk associated with any S-GA use and also with each individual S-GA. However, olanzapine of the S-GA drug group has the highest diabetogenic properties [26] and could therefore differ from the other S-GAs in its teratogenic potential. If our findings were related to maternal hyperglycemia, it is difficult to explain why previous studies have not shown an association between olanzapine use and an increased risk of overall congenital malformations or cardiac malformations. Maternal hyperglycemia is also an unlikely explanation, as we adjusted for pregestational and gestational diabetes in the analyses for overall malformations, and for pregestational diabetes in the analyses of organ specific malformations,
The discrepant findings compared to previous research might even be related to genetic differences in drug metabolizing enzyme activity across populations. Olanzapine is metabolized by hepatic glucuronidation and by cytochrome P450 (CYP) 1A2 and to a minor extent by CYP 2D6 -mediated oxidation to inactive metabolites. Ethnic differences exist for both CYP enzymes but CYP 1A2 activity does not differ significantly between the Finnish population and other Caucasians populations [27]. Further, the findings related to the impact of CYP1A2 and UDP-glucuronosyltransferase (UGT) 1A4 variants on olanzapine steady-state concentrations have been conflicting [28, 29]. The genetic profile of CYP2D6 in the Finnish population differs from North European populations in that the frequency of the ultrarapid metabolizer genotype is higher and that of poor metabolizer is lower in the Finnish population [30]. However, CYP2D6 polymorphism appears to have no significant influence on olanzapine pharmacokinetics [28, 29]. No further conclusions related to pharmacogenetic differences can be made as we had no genetic information on the subjects.
The risk of overall and musculoskeletal malformations in olanzapine exposed pregnancies was not statistically significantly increased when compared to F-GA users, suggesting that maternal illness or illness-related factors may have contributed to the findings. However, the risk estimates for olanzapine in these comparisons—even if not statistically significant -were strikingly different from the other S-GAs, showing a risk increase of round 30% for both overall and musculoskeletal malformations, while the risk estimates for other S-GAs were below or close to one.
Our study has several strengths. First, we included a comparison group of women exposed to F-GAs, controlling for maternal illness. Second—and contrary to previous studies—we included pregnancy terminations due to fetal malformation, which is important as more than 10% of all pregnancies where the fetus has been diagnosed with a major malformation are currently terminated [31]. Third, the reimbursement register includes 99% of all reimbursed medications [32], and the National Medical Birth Register data have been validated and are considered good [33]. Further, the data sources included in our study allowed for extensive control of potential confounders.
The limitations are those typical for studies using large administrative databases and national register data. Exposure misclassification may occur if the woman does not take her medication even if she bought it in the pharmacy, moving the risk estimate towards one if the drug was a teratogen. However, antipsychotic drugs are generally prescribed for psychotic conditions needing drug treatment and their use continues in a relatively constant manner from preconception through first trimester [34]. Residual confounding is an unlikely explanation to our findings as one would expect residual confounding to affect the results of all individual S-GAs and not only olanzapine.
We conclude that olanzapine use in early pregnancy is associated with an increased risk of overall malformations, and specifically musculoskeletal malformations. Until these results are either confirmed or refuted, use of olanzapine should only be used in pregnancy when no safer alternatives exist.
Availability of study material
The data that support the findings of this study are not publicly available. According to the national data protection legislation, permission to obtain the research data must be applied from the Finnish Institution for Health and Welfare.
References
Rhee TG, Olfson M, Nierenberg AA, Wilkinson ST (2020) 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry 177(8):706–715. https://doi.org/10.1176/appi.ajp.2020.19091000
Maher AR, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, Wang Z, Timmer M, Sultzer D, Shekelle PG (2011) Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA 306(12):1359–1369. https://doi.org/10.1001/jama.2011.1360
Reutfors J, Cesta CE, Cohen JM, Bateman BT, Brauer R, Einarsdóttir K, Engeland A, Furu K, Gissler M, Havard A, Hernandez-Diaz S, Huybrechts KF, Karlstad Ø, Leinonen MK, Li J, Man KKC, Pazzagli L, Schaffer A, Schink T, Wang Z, Yu Y, Zoega H, Bröms G (2020) Antipsychotic drug use in pregnancy: A multinational study from ten countries. Schizophr Res 220:106–115. https://doi.org/10.1016/j.schres.2020.03.048
Ellfolk M, Leinonen MK, Gissler M, Lahesmaa-Korpinen AM, Saastamoinen L, Nurminen ML, Malm H (2020) Second-generation antipsychotics and pregnancy complications. Eur J Clin Pharmacol 76(1):107–115. https://doi.org/10.1007/s00228-019-02769-z
Gjerden P, Bramness JG, Tvete IF, Slørdal L (2017) The antipsychotic agent quetiapine is increasingly not used as such: dispensed prescriptions in Norway 2004–2015. Eur J Clin Pharmacol 73(9):1173–1179. https://doi.org/10.1007/s00228-017-2281-8
Huybrechts KF, Hernández-Díaz S, Patorno E, Desai RJ, Mogun H, Dejene SZ, Cohen JM, Panchaud A, Cohen L, Bateman BT (2016) Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiat 73(9):938–946. https://doi.org/10.1001/jamapsychiatry.2016.1520
Vigod SN, Gomes T, Wilton AS, Taylor VH, Ray JG (2015) Antipsychotic drug use in pregnancy: high dimensional, propensity matched, population based cohort study. BMJ (Clinical research ed) 350:h2298. https://doi.org/10.1136/bmj.h2298
Damkier P, Videbech P (2018) The safety of second-generation antipsychotics during pregnancy: a clinically focused review. CNS Drugs 32(4):351–366. https://doi.org/10.1007/s40263-018-0517-5
Habermann F, Fritzsche J, Fuhlbrück F, Wacker E, Allignol A, Weber-Schoendorfer C, Meister R, Schaefer C (2013) Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. J Clin Psychopharmacol 33(4):453–462. https://doi.org/10.1097/JCP.0b013e318295fe12
Mitchell AA (2003) Systematic identification of drugs that cause birth defects–a new opportunity. N Engl J Med 349(26):2556–2559. https://doi.org/10.1056/NEJMsb031395
Perkins NJ, Cole SR, Harel O, Tchetgen Tchetgen EJ, Sun B, Mitchell EM, Schisterman EF (2018) Principled approaches to missing data in epidemiologic studies. Am J Epidemiol 187(3):568–575. https://doi.org/10.1093/aje/kwx348
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR (2009) Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ (Clinical research ed) 338:b2393. https://doi.org/10.1136/bmj.b2393
Graham JW, Olchowski AE, Gilreath TD (2007) How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 8(3):206–213. https://doi.org/10.1007/s11121-007-0070-9
Rubin D (1987) Multiple imputation for nonresponse in surveys. Wiley, New York. https://doi.org/10.1002/9780470316696
Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Müller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatr 61(9):540–560. https://doi.org/10.1177/0706743716659417
Hirschtritt ME, Bloch MH, Mathews CA (2017) Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA 317(13):1358–1367. https://doi.org/10.1001/jama.2017.2200
GDP (2019) Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol 74(5):596–607. https://doi.org/10.1037/amp0000473
National Institute for Health and Care Excellence (2018) Post-traumatic stress disorder. NICE guideline (NG116). https://www.nice.org.uk/guidance/ng116
Reinhard MA, Seifert J, Greiner T, Toto S, Bleich S, Grohmann R (2020) Pharmacotherapy of 1,044 inpatients with posttraumatic stress disorder: current status and trends in German-speaking countries. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-020-01223-x
Bandelow B, Michaelis S, Wedekind D (2017) Treatment of anxiety disorders. Dialogues Clin Neurosci 19(2):93–107. https://doi.org/10.31887/DCNS.2017.19.2/bbandelow
Thompson W, Quay TAW, Rojas-Fernandez C, Farrell B, Bjerre LM (2016) Atypical antipsychotics for insomnia: a systematic review. Sleep Med 22:13–17. https://doi.org/10.1016/j.sleep.2016.04.003
Källén B, Borg N, Reis M (2013) The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel, Switzerland) 6(10):1221–1286. https://doi.org/10.3390/ph6101221
Schaefer-Graf UM, Buchanan TA, Xiang A, Songster G, Montoro M, Kjos SL (2000) Patterns of congenital anomalies and relationship to initial maternal fasting glucose levels in pregnancies complicated by type 2 and gestational diabetes. Am J Obstet Gynecol 182(2):313–320. https://doi.org/10.1016/s0002-9378(00)70217-1
Towner D, Kjos SL, Leung B, Montoro MM, Xiang A, Mestman JH, Buchanan TA (1995) Congenital malformations in pregnancies complicated by NIDDM. Diabetes Care 18(11):1446–1451. https://doi.org/10.2337/diacare.18.11.1446
Ornoy A, Reece EA, Pavlinkova G, Kappen C, Miller RK (2015) Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today 105(1):53–72. https://doi.org/10.1002/bdrc.21090
Grajales D, Ferreira V, Valverde ÁM (2019) Second-generation antipsychotics and dysregulation of glucose metabolism: beyond weight gain. Cells 8(11). https://doi.org/10.3390/cells8111336
Hilli J, Rane A, Lundgren S, Bertilsson L, Laine K (2007) Genetic polymorphism of cytochrome P450s and P-glycoprotein in the Finnish population. Fundam Clin Pharmacol 21(4):379–386. https://doi.org/10.1111/j.1472-8206.2007.00494.x
Söderberg MM, Dahl ML (2013) Pharmacogenetics of olanzapine metabolism. Pharmacogenomics 14(11):1319–1336. https://doi.org/10.2217/pgs.13.120
Zubiaur P, Soria-Chacartegui P, Koller D, Navares-Gómez M, Ochoa D, Almenara S, Saiz-Rodríguez M, Mejía-Abril G, Villapalos-García G, Román M, Martín-Vílchez S, Abad-Santos F (2021) Impact of polymorphisms in transporter and metabolizing enzyme genes on olanzapine pharmacokinetics and safety in healthy volunteers. Biomed Pharmacother 133:111087. https://doi.org/10.1016/j.biopha.2020.111087
Pietarinen P, Tornio A, Niemi M (2016) High Frequency of CYP2D6 Ultrarapid Metabolizer Genotype in the Finnish Population. Basic Clin Pharmacol Toxicol 119(3):291–296. https://doi.org/10.1111/bcpt.12590
Finnish Institute of Health and Welfare THL (2017) Congenital anomalies. https://thl.fi/en/web/thlfi-en/statistics/statistics-by-topic/sexual-and-reproductive-health/congenital-anomalies
Finnish Statistics on Medicines 2018 (2019). http://urn.fi/URN:NBN:fi-fe2019123149481
Gissler M, Teperi J, Hemminki E, Meriläinen J (1995) Data quality after restructuring a national medical registry. Scand J Soc Med 23(1):75–80. https://doi.org/10.1177/140349489502300113
Malm H, Martikainen J, Klaukka T, Neuvonen PJ (2003) Prescription drugs during pregnancy and lactation–a Finnish register-based study. Eur J Clin Pharmacol 59(2):127–133. https://doi.org/10.1007/s00228-003-0584-4
Acknowledgements
The Drugs and Pregnancy working group, THL, Kela, and FIMEA.
Funding
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. The Drugs and Pregnancy project is financed by the Finnish Medicines Agency (FIMEA), the Finnish Institute for Health and Welfare (THL), and the Social Insurance Institution in Finland (Kela). Funding for this study has been granted from the Helsinki University and Helsinki University Hospital, Department of Emergency care, Helsinki, Finland.
Author information
Authors and Affiliations
Contributions
Maria Ellfolk: conceptualization, methodology, writing—original draft preparation. Maarit K Leinonen: methodology, data curation, software, statistics, validation, writing and editing. Mika Gissler: methodology, data curation, software, statistics, writing and editing. Sonja Kiuru-Kuhlefelt: methodology, data curation, data curation of congenital malformations, writing and editing. Leena Saastamoinen: conceptualization, methodology, writing—original draft preparation. Heli Malm: conceptualization, methodology, writing—original draft preparation, reviewing and editing, supervision.
Corresponding author
Ethics declarations
Ethics approval
The utilization of sensitive health register data for scientific research and the data linkages in the “Drugs and Pregnancy” project have been approved by the register administrators and the national data protection authority. The study protocol was approved by the Institutional Review Board at Finnish Institute for Health and Welfare (THL).
Consent to participate
Since the study subjects are not contacted, according to the Finnish legislation informed consent is not required for large register studies.
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ellfolk, M., Leinonen, M.K., Gissler, M. et al. Second-generation antipsychotic use during pregnancy and risk of congenital malformations. Eur J Clin Pharmacol 77, 1737–1745 (2021). https://doi.org/10.1007/s00228-021-03169-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03169-y