Abstract
Purpose
Comorbid conditions of heart and liver disorders added to HCV-induced hepatic steatosis make co-administration of statins, and direct-acting antivirals is common in clinical practice. This study aimed to evaluate the pharmacokinetic interaction of atorvastatin and fixed-dose combination of sofosbuvir/ledipasvir “FDCSL” with rationalization to the underlying mechanism.
Methods
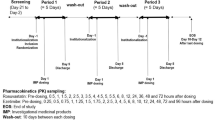
A randomized, three-phase crossover study that involves 12 healthy volunteers was performed. Participants received a single-dose of atorvastatin 80 mg alone, atorvastatin 80-mg plus tablets containing 400/90 mg FDCSL, or tablets containing 400/90 mg FDCSL alone. Plasma samples were analyzed using liquid chromatography–tandem mass spectrometry (LC–MS/MS) for atorvastatin, sofosbuvir, ledipasvir, and sofosbuvir metabolite “GS-331007,” and their pharmacokinetics parameters were determined.
Results
Compared to atorvastatin alone, the administration of FDCSL caused a significant increase in both areas under the concentration–time curve from time zero to infinity (AUC0−∞) and maximum plasma concentration (Cmax) of atorvastatin by 65.5% and 156.0%, respectively. Also, atorvastatin caused a significant increase in the AUC0−∞ and Cmax of sofosbuvir by 32.0% and 11.0%, respectively. Similarly, AUC0−∞ and Cmax of sofosbuvir metabolite significantly increased by 84.0% and 74.0%, respectively. However, ledipasvir AUC0−∞ showed no significant change after atorvastatin intake. The elimination rate in all drugs revealed no significant changes.
Conclusion
After concurrent administration of FDCSL with atorvastatin, the AUC0−∞ of both atorvastatin and sofosbuvir were increased. Caution should be taken with close monitoring for possible side effects after co-administration of atorvastatin and FDCSL in clinical practice.




Similar content being viewed by others
References
Abd-Elsalam S, Badawi R, Elnawasany S, Yousef M, Mansour L, Hawash N, Elkhouly RA, Soliman S, Selim A, Kobtan A (2019) Sofosbuvir, pegylated interferon and ribavirin in the treatment of an Egyptian cohort with hepatitis C virus infection in real-life clinical practice. Infect Disord Drug Targets (formerly current drug targets-infectious disorders) 19(2):179–184. https://doi.org/10.2174/1871526518666180912121835
Ahmed OA, Kaisar HH, Badawi R, Hawash N, Samir H, Shabana SS, Fouad MHA, Rizk FH, Khodeir SA, Abd-Elsalam S (2018) Efficacy and safety of sofosbuvir–ledipasvir for treatment of a cohort of Egyptian patients with chronic hepatitis C genotype 4 infection. Infect Drug Resist 11:295–298. https://doi.org/10.2147/IDR.S153060
Cha A, Budovich A (2014) Sofosbuvir: a new oral once-daily agent for the treatment of hepatitis C virus infection. Pharm Ther 39(5):345–352
Kim CW, Chang K-M (2013) Hepatitis C virus: virology and life cycle. Clin Mol Hepatol 19(1):17–25. https://doi.org/10.3350/cmh.2013.19.1.17
Elgharably A, Gomaa AI, Crossey MM, Norsworthy PJ, Waked I, Taylor-Robinson SD (2017) Hepatitis C in Egypt–past, present, and future. Int J Gen Med 10:1–6. https://doi.org/10.2147/IJGM.S119301
Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE (2014) Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet 383(9916):515–523. https://doi.org/10.1016/S0140-6736(13)62121-2
Stambouli O (2014) Hepatitis C virus: molecular pathways and treatments. Published by OMICS Group eBooks, pp47–48
Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R (2014) Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 370(16):1483–1493. https://doi.org/10.1056/NEJMoa1316366
Wei L, Xie Q, Hou JL, Tang H, Ning Q, Cheng J, Nan Y, Zhang L, Li J, Jiang J (2018) Ledipasvir/sofosbuvir for treatment-naive and treatment-experienced Chinese patients with genotype 1 HCV: an open-label, phase 3b study. Hepatol Int 12(2):126–132. https://doi.org/10.1007/s12072-018-9856-z
Kattakuzhy S, Levy R, Kottilil S (2015) Sofosbuvir for treatment of chronic hepatitis C. Hepatol Int 9(2):161–173. https://doi.org/10.1007/s12072-014-9606-9
Cholongitas E, Papatheodoridis GV (2014) Sofosbuvir: a novel oral agent for chronic hepatitis C. Ann Gastroenterol 27(4):331–337
Kirby BJ, Symonds WT, Kearney BP, Mathias AA (2015) Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin Pharmacokinet 54(7):677–690. https://doi.org/10.1007/s40262-015-0261-7
Li X, Liu Y, Xu B, Liu L, Li Y, Zhang P, Wang Y (2020) Evaluation of the pharmacokinetics and food intake effect of generic sofosbuvir in healthy Chinese subjects. Int J Clin Pharmacol Ther 58(4):230–241. https://doi.org/10.5414/cp203649
Noell BC, Besur SV, Andrew S (2015) Changing the face of hepatitis C management–the design and development of sofosbuvir. Drug Des Dev Ther 9:2367–2374. https://doi.org/10.2147/DDDT.S65255
German P, Mathias A, Brainard D, Kearney BP (2016) Clinical pharmacokinetics and pharmacodynamics of ledipasvir/sofosbuvir, a fixed-dose combination tablet for the treatment of hepatitis C. Clin Pharmacokinet 55(11):1337–1351. https://doi.org/10.1007/s40262-016-0397-0
German P, Mathias A, Brainard DM, Kearney BP (2018) Drug–Drug Interaction Profile of the Fixed-Dose Combination Tablet Regimen Ledipasvir/Sofosbuvir. Clin Pharmacokinet 57(11):1369–1383. https://doi.org/10.1007/s40262-018-0654-5
El-Sisi A, Hegazy S, Salem K, AbdElkawy K (2013) Atorvastatin improves erectile dysfunction in patients initially irresponsive to sildenafil by the activation of endothelial nitric oxide synthase. Int J Impot Res 25(4):143–148. https://doi.org/10.1038/ijir.2012.46
Koskinas KC, Windecker S, Räber L (2016) Regression of coronary atherosclerosis: current evidence and future perspectives. Trends Cardiovasc Med 26(2):150–161. https://doi.org/10.1016/j.tcm.2015.05.004
Tournadre A (2020) Statins, myalgia, and rhabdomyolysis. Joint Bone Spine 87(1):37–42. https://doi.org/10.1016/j.jbspin.2019.01.018
Janicko M, Drazilova S, Pella D, Fedacko J, Jarcuska P (2016) Pleiotropic effects of statins in the diseases of the liver. World J Gastroenterol 22(27):6201–6213. https://doi.org/10.3748/wjg.v22.i27.6201
O'Leary JG, Chan JL, McMahon CM, Chung RT (2007) Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology 45(4):895–898. https://doi.org/10.1002/hep.21554
Henderson LM, Patel S, Giordano TP, Green L, El-Serag HB (2010) Statin therapy and serum transaminases among a cohort of HCV-infected veterans. Dig Dis Sci 55(1):190–195. https://doi.org/10.1007/s10620-009-0959-1
Pedersen MR, Patel A, Backstedt D, Choi M, Seetharam AB (2016) Genotype specific peripheral lipid profile changes with hepatitis C therapy. World J Gastroenterol 22(46):10226–10231. https://doi.org/10.3748/wjg.v22.i46.10226
Pan HY, DeVault AR, Wang-Iverson D, Ivashkiv E, Swanson BN, Sugerman AA (1990) Comparative pharmacokinetics and pharmacodynamics of pravastatin and lovastatin. J Clin Pharmacol 30(12):1128–1135. https://doi.org/10.1002/j.1552-4604.1990.tb01856.x
Lennernäs H (2003) Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet 42(13):1141–1160. https://doi.org/10.2165/00003088-200342130-00005
Watanabe T, Kusuhara H, Maeda K, Kanamaru H, Saito Y, Hu Z, Sugiyama Y (2010) Investigation of the rate-determining process in the hepatic elimination of HMG-CoA reductase inhibitors in rats and humans. Drug Metab Dispos 38(2):215–222. https://doi.org/10.1124/dmd.109.030254
Black AE, Hayes RN, Roth BD, Woo P, Woolf TF (1999) Metabolism and excretion of atorvastatin in rats and dogs. Drug Metab Dispos 27(8):916–923
Knopp RH (1999) Drug treatment of lipid disorders. N Engl J Med 341(7):498–511. https://doi.org/10.1056/NEJM199908123410707
Roingeard P (2013) Hepatitis C virus diversity and hepatic steatosis. J Viral Hepat 20(2):77–84. https://doi.org/10.1111/jvh.12035
Ikeda M, Ki A, Yamada M, Dansako H, Naka K, Kato N (2006) Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology 44(1):117–125. https://doi.org/10.1002/hep.21232
Rickham PP (1964) Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. Br Med J 2(5402):177–177. https://doi.org/10.1136/bmj.2.5402.177
Food F-U (2001) Guidance for industry-bioanalytical method validation.available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf.
Cohen J (1992) A power primer. Psychol Bull 112(1):155–159
Foster City C, USA: Gilead Sciences (2015) Harvoni (ledipasvir-sofosbuvir) tablets: U.S. prescribing information. https://gilead2019tf.q4web.com/files/doc_news/archive/f4e0ab1f-deb4-47b2-adf6-3194603ef93c.pdf
FDA (2014) Food and Drug Administration FDA approves first combination pill to treat hepatitis C. https://www.fda.gov/news-events/press-announcements/fda-approves-two-hepatitis-c-drugs-pediatric-patients
Wilkinson GR, Shand DG (1975) A physiological approach to hepatic drug clearance. Clin Pharmacol Ther 18(4):377–390
Kantola T, Kivistö KT, Neuvonen PJ (1998) Effect of itraconazole on the pharmacokinetics of atorvastatin. Clin Pharmacol Ther 64(1):58–65. https://doi.org/10.1016/S0009-9236(98)90023-6
Lau Y, Huang Y, Frassetto L, Benet L (2007) Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther 81(2):194–204. https://doi.org/10.1038/sj.clpt.6100038
German P, Pang P, West S, Han L, Sajwani K, Mathias A (2014) Drug interactions between direct acting anti-HCV antivirals sofosbuvir and ledipasvir and HIV antiretrovirals. In: 15th international workshop on clinical pharmacology of HIV and hepatitis therapy. Washington DC, May, pp 19–21
Bellesini M, Bianchin M, Corradi C, Donadini MP, Raschi E, Squizzato A (2020) Drug–drug interactions between direct oral anticoagulants and hepatitis C direct-acting antiviral agents: looking for evidence through a systematic review. Clin Drug Investig 40:1001–1008. https://doi.org/10.1007/s40261-020-00962-y
Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB (2006) Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology 130(6):1793–1806. https://doi.org/10.1053/j.gastro.2006.02.034
Patel S, Andres J, Qureshi K (2016) An unexpected interaction between sofosbuvir/ledipasvir and atorvastatin and colchicine causing rhabdomyolysis in a patient with impaired renal function. Case Rep Med 2016:3191089. https://doi.org/10.1155/2016/3191089
Li S, Yu Y, Jin Z, Dai Y, Lin H, Jiao Z, Ma G, Cai W, Han B, Xiang X (2019) Prediction of pharmacokinetic drug-drug interactions causing atorvastatin-induced rhabdomyolysis using physiologically based pharmacokinetic modelling. Biomed Pharmacother 119:109416. https://doi.org/10.1016/j.biopha.2019.109416
Minami K, Higashino H, Kataoka M, Yamashita S (2020) Species differences in the drug–drug interaction between atorvastatin and cyclosporine: In vivo study using a stable isotope-IV method in rats and dogs. Eur J Pharm Sci 152:105409. https://doi.org/10.1016/j.ejps.2020.105409
Ankrom W, Sanchez RI, Yee KL, Fan L, Mitra P, Wolford D, Triantafyllou I, Sterling LM, Stoch SA, Iwamoto M (2019) Investigation of pharmacokinetic interactions between doravirine and elbasvir-grazoprevir and ledipasvir-sofosbuvir. Antimicrob Agents Chemother 63(5):e02491–e02418. https://doi.org/10.1128/AAC.02491-18
Davis P(2014) LIPITOR(R) oral tablets, atorvastatin calcium oral tablets. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020702s056lbl.pdf
Goard CA, Mather RG, Vinepal B, Clendening JW, Martirosyan A, Boutros PC, Sharom FJ, Penn LZ (2010) Differential interactions between statins and P-glycoprotein: implications for exploiting statins as anticancer agents. Int J Cancer 127(12):2936–2948. https://doi.org/10.1002/ijc.25295
Gogman K, Peyer A, Torok M, Lusters E, Drewe J (2001) HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br J Pharmacol 132:1183–1192. https://doi.org/10.1038/sj.bjp.0703920
Stage TB, Mortensen C, Khalaf S, Steffensen V, Hammer HS, Xiong C, Nielsen F, Poetz O, Svenningsen ÅF, Rodriguez-Antona C (2020) P-glycoprotein inhibition exacerbates paclitaxel neurotoxicity in neurons and patients with cancer. Clin Pharmacol Ther 108:671–680
Wang E-j, Casciano CN, Clement RP, Johnson WW (2001) HMG-CoA reductase inhibitors (statins) characterized as direct inhibitors of P-glycoprotein. Pharm Res 18(6):800–806. https://doi.org/10.1023/A:1011036428972
Jacobsen W, Kuhn B, Soldner A, Kirchner G, Sewing K-F, Kollman PA, Benet LZ, Christians U (2000) Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab Dispos 28(11):1369–1378
Sakaeda T, Fujino H, Komoto C, Kakumoto M, J-s J, Iwaki K, Nishiguchi K, Nakamura T, Okamura N, Okumura K (2006) Effects of acid and lactone forms of eight HMG-CoA reductase inhibitors on CYP-mediated metabolism and MDR1-mediated transport. Pharm Res 23(3):506–512. https://doi.org/10.1007/s11095-005-9371-5
Boyd RA, Stern RH, Stewart BH, Wu X, Reyner EL, Zegarac EA, Randinitis EJ, Whitfield L (2000) Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion. J Clin Pharmacol 40(1):91–98. https://doi.org/10.1177/009127000004000112
Denning J, Cornpropst M, Flach SD, Berrey MM, Symonds WT (2013) Pharmacokinetics, safety, and tolerability of GS-9851, a nucleotide analog polymerase inhibitor for HCV, following single ascending doses in healthy subjects. Antimicrob Agents Chemother 57(3):1201–1208. https://doi.org/10.1128/AAC.01262-12
Acknowledgements
Authors thank the members of the Clinical Pharmacy Department, Faculty of Pharmacy, Kafrelsheikh University.
Data availability (data transparency)
The datasets generated during and/or analyzed during the current study are not publicly available due to confidentiality reasons but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Conceptualization: FE, HE, and KA
Methodology: HE, FB, KA, and AEA
Validation: FE, FB, AAA, and KA
Formal analysis: FE, HE, AEA, and AAA
Investigation: FE, HE, and KA
Resources: FE, HE, and KA
Data curation: FE, HE, KA, AEA, and AAA
Writing—original draft preparation: HE, KA, and FE
Writing—review and editing: FE, HE, KA, AEA, and AAA
Supervision: FE, KA
Project administration: KA, HE, and FE
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study protocol was approved by the ethics committee of Kafrelsheikh University in accordance with the Declaration of Helsinki and its amendments.
Informed consent
All subjects provided written informed consent before participation.
Consent for publication
Patients expressed no objection for the publication of the results.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Atorvastatin increases the extent of absorption of sofosbuvir and its metabolite (GS-331007) without affecting ledipasvir pharmacokinetics.
• Atorvastatin AUC0−∞ and Cmax increased after FDCSL intake.
• Patients should take caution about atorvastatin and sofosbuvir side effects.
Rights and permissions
About this article
Cite this article
Elmekawy, H.A., Belal, F., Abdelaziz, A.E. et al. Pharmacokinetic interaction between atorvastatin and fixed-dose combination of sofosbuvir/ledipasvir in healthy male Egyptian volunteers. Eur J Clin Pharmacol 77, 1369–1379 (2021). https://doi.org/10.1007/s00228-021-03130-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03130-z