Abstract
Purpose
Computerised prescriber (or physician) order entry (CPOE) implementation is one of the strategies to reduce medication errors. The extent to which CPOE influences the incidence of chemotherapy-related medication errors (CMEs) was not previously collated and systematically reviewed. Hence, this study was designed to collect, collate, and systematically review studies to evaluate the effect of CPOE on the incidence of CMEs.
Methods
A search was performed of four databases from 1 January 1995 until 1 August 2019. English-language studies evaluating the effect of CPOE on CMEs were selected as per inclusion and exclusion criteria. The total CMEs normalised to total prescriptions pre- and post-CPOE were extracted and collated to perform a meta-analysis using the ‘meta’ package in R. The systematic review was registered with PROSPERO CRD42018104220.
Results
The database search identified 1621 studies. After screening, 19 studies were selected for full-text review, of which 11 studies fulfilled the selection criteria. The meta-analysis of eight studies with a random effects model showed a risk ratio of 0.19 (95% confidence interval: 0.08–0.44) favouring CPOE (I2 = 99%).
Conclusion
The studies have shown consistent reduction in CMEs after CPOE implementation, except one study that showed an increase in CMEs. The random effects model in the meta-analysis of eight studies showed that CPOE implementation reduced CMEs by 81%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medication error (ME) is defined as any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care products, procedures, and systems, including prescribing, order communication, product labelling, packaging, nomenclature, compounding, dispensing, distribution, administration, education, monitoring, and use [1]. ME is the ‘inappropriate use of a drug that may or may not result in harm’, and any harm occurring as a result of a ME is termed an adverse drug event (ADE) [2]. It is estimated that one-third of all hospital adverse events are attributed to ADEs; thus, drug safety significantly determines patient safety [3]. The World Health Organization (WHO) aptly launched in 2017 the third Global Patient Safety Challenge, ‘Medication Without Harm’ to reduce avoidable medication errors in all countries by over 50% by 2022 [4].
MEs in oncology have a greater impact on patient lives as cancer patients are vulnerable, and many chemotherapeutic agents exhibit a narrow therapeutic index [5]. The Institute of Safe Medication Practices has placed cancer chemotherapeutics at the top of the list of high-alert medications in acute, ambulatory, and long-term care settings [6]. MEs, such as dosing calculation errors, contribute significantly to the burden of ADEs, resulting in increased morbidity and mortality with additional economic impacts [7].
Chemotherapy-related medication errors (CMEs) affect 1–3% of oncology patients and occur in all phases of drug use, compromising safety [8, 9]. Previously, we reported rates of CMEs during the prescription, preparation, dispensing, and administration phases were 0.1–24.6%, 0.4–0.5%, 0–0.03%, and 0.02–0.1% of the total orders, respectively [10]. The prescription phase is highly vulnerable to the occurrence of errors that are largely preventable via strategies such as the implementation of computerised prescriber (or physician) order entry (CPOE). CPOE has shown promising results in preventing CMEs and improving safety in patients receiving complex chemotherapy regimens [11]. Although there are several reports on the utility of CPOE in improving patient safety in a chemotherapy setting, to our knowledge, no reports have collated and systematically reviewed the extent to which CPOE influences the occurrence of CMEs. Hence, this study was designed to collect, collate, and systematically review studies to evaluate the impact of CPOE implementation on the incidence of CMEs.
Methods
Search strategy
A search was performed using keywords such as ‘computerised physician order entry’, ‘computerised provider order entry’, ‘computerised prescriber order entry’, ‘CPOE and chemotherapy’, and ‘chemotherapy medication errors’. Using this search strategy (Supplementary Material for Search Tree), we explored the Medline, Web of Science, Agency for Healthcare Research and Quality, and Cumulative Index to Nursing and Allied Health Literature databases from 1 January 1995 to 1 August 2019. Three authors (SKS, RA, and SK) independently searched each database. The data collected from all databases were combined and screened for any duplication of records. Subsequently, the abstracts of all the relevant studies were reviewed for eligibility. During the full-text screening, the cited references were further scrutinised for relevance. This systematic review conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting [12].
Eligibility criteria
The inclusion criteria were as follows: (a) studies evaluating the effect of CPOE on CMEs, (b) studies providing numbers of CMEs with respect to the total number of prescriptions pre-and post-CPOE implementation, and (c) publications only in the English language. Studies evaluating multiple interventions and without non-intervention groups were excluded. Discrepancies regarding article inclusion and exclusion were resolved by discussion among all the screening and reviewing authors. Where necessary, the authors of the original reports were contacted for data on prescriptions for the analysis.
Data extraction, quality assessment, and statistical analysis
Two reviewers (SKS and RA) extracted the data (Table 1). Using 13 different criteria adopted from previous reports, quality assessments were performed on all the studies included in the review [13, 14]. The criteria included reporting clearly described objectives, errors, error definitions, error categories, denominators, methodologies, settings, sample size calculations, reliabilities, validities, assumptions, limitations, and ethics committee approval. The quality scores were used to determine the overall applicability and impact of the studies, as well as to define the eligibility of studies for the meta-analysis. The extracted outcome data (i.e. risk ratios estimated using the Mantel–Haenszel method for the occurrence of CMEs pre- and post-CPOE) were collated to calculate the pooled estimates. A meta-analysis (random effects model) was performed using the ‘meta’ package in the R statistical software version 3.6.2 [15, 16]. Variance estimation (tau-squared) for the distribution of the true effect sizes was calculated using the DerSimonian–Laird estimator [17]. Publication bias was visualised using a funnel plot.
Results
Study selection
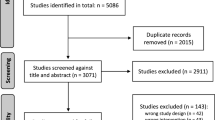
Of the 1621 studies identified by the search, 34 duplicate and 1572 unrelated reports were removed. The remaining 15 full-text articles plus four additional articles cited in these papers were reviewed. Among the 19 studies, eight did not meet the eligibility criteria [18,19,20,21,22,23,24,25]. Thus, 11 studies were eligible and received a consensus as shown in Fig. 1. Among the excluded studies that did not meet inclusion criteria, one descriptive study provided data of post-CPOE alone [18]. Another study evaluated duplicate checks in a non-chemotherapy setting [19]. Two studies did not mention total number of prescriptions, and we were unable to retrieve the data by correspondence with the authors [20, 21]. Two studies were comparative prospective parallel studies [22, 23]. One prospective audit reported CPOE vs. spread sheet [24]. One study compared two methods of order entry within CPOE [25].
Study characteristics
All 11 studies were published between 2006 and 2018 and had a single-arm design in which the CPOE pre-and post-implementation phases were compared [26,27,28,29,30,31,32,33,34,35,36]. Among these, five studies were from the USA, three from Europe, and three from Asian countries. The extracted data on CME events and the study parameters are shown in Tables 1 and 2. The average CME event rate (number of events/total number of prescriptions, in percentage) pre-CPOE implementation was 20.7% (range: 1.8–100%) and was reduced to 5.12% (range: 0.26–25.0%) post-CPOE. Nine studies have shown statistically significant reductions in CMEs post-CPOE compared to pre-CPOE (Table 2). Significant reductions in CMEs related to medical devices, adjuvants, infusion rates, and routes of administration have been documented [26, 27]. However, in one of the included studies, there was a statistically significant increase in a specific type of CME—i.e. a mismatch between orders and treatment plans post-CPOE [RR 5.4 (CI 3.1–9.5)]—that affected the total CME events [26].
The different types of CPOE used in the studies are listed in Table 2. One of the studies reported complete elimination of all CMEs with adequate training and acclimatisation of personnel over a period of 5 years [33]. Two studies did not provide inferential statistics; however, the number of events pre- and post-CPOE differed significantly (Table 2, P<0.05) [28, 34]. The average quality score of the included studies was 7.2 with 95% CI (6.08–8.12) (Supplementary Table 1).
Healthcare Failure Mode and Effects Analysis (HFMEA) or Failure Mode and Effects Analysis (FMEA) showed reductions in CMEs post-CPOE in four of the included studies [26, 29, 30, 35]. HFMEA strategies have been adopted in conjunction with CPOE with additional alerting modules for pathological conditions such as renal and liver dysfunction to execute dose modifications accordingly [30]. CPOE was also shown to reduce CMEs when complemented with supporting systems such as complete prescription audit systems (CPASs) [35] and clinical decision support systems (CDSSs) [34].
The maximum numbers of prescriptions were evaluated by two studies having a quality score of 8 [28, 31]. Markert et al. demonstrated a reduction in CMEs (including patient data errors) during the prescription phase from 8.5% to 7.4% following CPOE [28]. In the same report, CMEs in outpatients decreased from 4% to 2.8% following CPOE, whereas for inpatients, it remained unchanged (4.4% vs. 4.7%) [28]. The study showed that the presence of a multidisciplinary clinical service centre (CSC) prevented 99.92% of all CMEs reaching the patients. The patients’ risk of experiencing a CME was estimated to be 0.13% of the total treated patients. However, the incidence of serious adverse events (SAEs) per patient per year was reported at 7.5% and 7.4%, respectively, pre- and post-CPOE (Table 2) [28]. Elsaid et al. have also reported error rates during three phases: pre-implementation (30 months), implementation (32 months), and post-implementation (28 months) [31]. The prescribing errors that were prevented per 1000 doses during pre-implementation, implementation, and post-implementation were 17.8, 9.1, and 7.9, respectively. The study showed that CPOE reduces CMEs with the highest effect being on dosing calculation errors, which were reduced by 94% [31].
Meisenberg et al. reported CMEs from three sequential patterns of prescription orders: handwritten (30.6%), preprinted (12.6%), and CPOE (2.2%) [32]. The harmful CMEs among the reported CMEs also showed a statistically significant decrease from handwritten (4.2%) to preprinted (1.5%) and CPOE (0.1%). This retrospective study involved analysing every 10th order of the handwritten and preprinted orders and every fifth order during the CPOE phase [32]. Collins et al. reported a 69% reduction in prescription errors for oral chemotherapy medication within 6 months post-CPOE [29]. In addition to reporting CMEs, one study reported improvement in dispensing and administration time with the use of CPOE. The mean administration time was reduced from 132 min (pre-CPOE) to 112 min (post-CPOE) [34].
User satisfaction was surveyed in three studies [34,35,36]. Aziz et al. showed that residents, consultants, and pharmacists perceived CPOE to be user friendly, while nurses perceived it as not user-friendly [34]. High satisfaction scores were reported for all end users by Wang et al. [35]. Chung et al., however, reported higher user satisfaction in one centre with the moderate dissatisfaction in two centres attributed to the problems in acclimatisation with the new system [36]. However, overall, no difference in satisfaction scores before and after CPOE was reported [36].
Three studies were prospectively designed to collect data from the prescription validation process while implementing CPOE [28, 33, 34], while in five studies, the data were collected as part of the routine quality assurance process and analysed retrospectively [26, 29,30,31,32]. The data collection methods were not clearly explained in three studies [27, 35, 36].
Seven studies reported clinical implications of CPOE on the occurrence of serious or fatal events among CMEs [27,28,29, 31, 32, 34, 35]. The major, fatal, or serious adverse events (SAEs) that ranged from 0.8 to 36.5% of CMEs pre-CPOE were reduced from 0% to 20% post-CPOE (Table 2). Adverse events with clinical implications were completely eliminated in three studies [27, 29, 31]. In one study, the SAE numbers remained unchanged pre- and post-CPOE; however, the SAE incidence per order per year decreased marginally from 0.8% to 0.76% [28]. Serious and fatal events reduced post-CPOE in another study from 36.5% to 20% [34].
Meta-analysis
The total number of CMEs reported with respect to the total number of prescriptions pre- and post-CPOE implementation was collated in the meta-analysis (Fig. 2). Studies with quality scores below the lower limit of 95% CI (≤ 6) were excluded from the meta-analysis [30, 35, 36]. Among these, one case study reported data from ‘selected’ prescriptions only that might have incorporated bias [36]. The favourable pooled effect with CPOE implementation resulted in an 81% reduction in CMEs. A pooled risk ratio (RR) of 0.19 (95% CI: 0.08–0.44) was observed favouring CPOE (random effects model, I2 = 99%) implementation (Fig. 2). The funnel plot was asymmetrical, indicating the presence of bias due to heterogeneity among the included studies (Supplementary Figure 1). Further investigations on asymmetry were not conducted as the number of studies included in the meta-analysis was below 10.
Forest plot of the studies reporting chemotherapy related medication errors pre- and post-CPOE implementation. The vertical line represents ‘line of no effect’. The post-CPOE data is shown on the left side of the vertical line, whereas the right side represents pre-CPOE data. X-axis represents relative risks
Discussion
To our knowledge, this systematic review represents the first effort to amalgamate available data on CME occurrence pre-and post-CPOE implementation (Table 1). Our analysis showed that CPOE implementation resulted in a significant reduction in CMEs (81%), indicating that it is a valuable strategy that can be used to reduce CMEs (Table 1; Fig. 2). The beneficial effects of CPOE have been previously reported in a descriptive study [37]. Similarly, CPOE implementation was shown to reduce 76% of MEs in all hospitalised patients [38] and by 85% in the intensive care unit patients [39].
Although CPOE implementation is valuable, it requires constant monitoring and training, especially during the initial implementation [18, 26]. The study by Kim et al. highlighted the need to link computerised treatment protocols with drug protocols to prevent CMEs [26]. The statistically significant increase in the non-matching of orders to treatment plans post-CPOE emphasised the need for proper preparation and constant surveillance, even post-CPOE [26]. Meisenberg et al. described CPOE (Beacon system)–related errors, such as unintended re-escalation of doses if the wrong cycle was copied while creating additional cycles; confusion while adding or deleting drug in a regimen without changing the title; retention of supportive medications even after the deletion of chemotherapy drugs; chances of overdosing when treatment days were reduced as the data was automatically updated for future cycles; and inappropriate omission of drugs if the prescriber forgot to sign the order [32].
Nevertheless, CPOE systems have evolved to offer solutions for such problems, e.g. adoption of HFMEA strategies which included additional alerting modules for pathological conditions such as liver dysfunction, enabling the execution of dose modifications, accordingly [30]. The US Joint Commission on Accreditation of Healthcare Organizations also advocates the use of HFMEA to improve patient safety [40]. CPOE also functioned well with complementary supporting systems such as CPASs [35] and CDSSs [36]. CPOE combined with an integrated CDSS, especially with artificial intelligence, could be an effective approach to medication safety [41, 42]. Importantly, CDSS modules customised to chemotherapy settings should be compliant with chemotherapy protocols, dose calculations, and dose adjustments. Furthermore, they must have provisions in place for alerts at the crucial juncture of prescribing and mandatory items to ensure completeness of the prescription process [43].
The data on the impact of CPOE on clinical outcomes were included in seven studies (Table 2) [27,28,29, 31, 32, 34, 35]. The magnitude of the reduction in the CMEs was translated to a reduction in adverse events, as shown by three studies where major, serious, and fatal adverse events were completely eliminated post-CPOE [27, 29, 31]. This is in agreement with a meta-analysis of reports from hospital-based settings which observed a nearly 50% reduction in preventable ADRs and medication errors (RR = 0.46; 95% CI: 0.35–0.60) upon CPOE implementation [44]. Aziz et al. showed a decrease in fatal and serious events post-CPOE [34]. However, Markert et al. reported an unchanged total number of SAEs pre- and post-CPOE, and a marginal decrease in SAE incidence per order per year [28]. The harmful errors were normalised to the total orders in the report by Meisenberg et al. [32]. However, there was no proper distinction between errors and adverse reactions in the findings of Wang et al. [35]. Thus, future studies designed to report CMEs should also include data on SAEs, which have important clinical implications.
Furthermore, CPOE improved the completeness of documentation and user satisfaction in outpatient oncology settings [45]. Thus, with respect to medication safety, CPOE is a structural asset in sensitive healthcare settings, including oncology and onco-haematology units. CPOE implementation should involve a multidisciplinary team of physicians, nurses, pharmacists, clinical pharmacologists, and information technology (IT) professionals. CPOE systems can eliminate MEs completely and can yield good results in an optimum time of 5 years after complete adaptation [33].
Our review should be viewed in light of the following strengths and limitations. The included studies were from different parts of the globe; hence, the results have generalisability and applicability. Most of the studies included were single-centre studies except for one study [36], which compromised the external validity (Table 1). Although single-arm designs have several limitations, they provide preliminary evidence of the effect in most scenarios. The data reported from the included studies of chemotherapy settings also included MEs from supportive care therapy, which are used along with chemotherapy. Nevertheless, all studies used different CPOE systems and in different settings; thus, the external validity of studies should be gauged. Our meta-analysis showed high heterogeneity (Fig. 2) due to the differences in hospital settings, reporting time periods, patient populations, sample sizes, data collection methods, and CPOE types (Table 1) and the definitions used to demarcate CMEs among the limited number of studies included. The high heterogeneity observed among studies limited their comparability that is commonly seen in studies reporting MEs as reported earlier [10].
The average quality scores of the studies included in this review (n=11) and meta-analysis (n=8) were 7.2 and 8.0, respectively, out of 13. Although most of the studies were poor in reporting reliability and validity measures, those excluded from meta-analysis scored poorly on study objectives, error definitions, error categories, and data collection methodologies. We also propose the implementation of and adherence to comprehensive checklist/uniform standards while reporting ME so that studies would be comparable.
In conclusion, a systematic review of 11 studies showed consistent reduction of CMEs after the implementation of CPOE. However, one study showed an increase in CMEs, which was attributed to improper preparation and acclimatisation. CPOE implementation reduced CMEs by 81% in a meta-analysis of eight studies. Thus, CPOE could be an effective strategy for limiting CMEs, provided that multidisciplinary approach to training and acclimatisation is provided.
Data availability
The study protocol was registered with PROSPERO CRD42018104220. All other relevant data mentioned in the article are provided in the supplementary material.
References
National Coordinating Council for Medication Error Reporting and Prevention (2014). What is a medication error? New York, NY: National Coordinating Council for Medication Error Reporting and Prevention. Available at http://www.nccmerp.org/ about-medication-errors. Accessed 4 October, 2020
Nebeker JR, Barach P, Samore MH (2004) Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med 140:795–801
U.S. Department of Health and Human Services Office of Inspector General (OIG). Adverse events in hospitals: national incidence among Medicare beneficiaries. Washington, DC. 2010 November. Report No.: OEI-06-09-00090
World health organisation (2017) WHO launches global effort to halve medication-related errors in 5 years. WHO, Geneva Available at: https://www.who.int/news-room/detail/29-03-2017-who-launches-global-effort-to-halve-medication-related-errors-in-5-years. Accessed 17 April 2020
Joshi MC (2007) Cytotoxic drug: towards safer chemotherapy practices. Indian J Cancer 44:31–35
Institute For Safe Medication Practices (2018) ISMP list of high alert medications. Available at: https://www.ismp.org/sites/default/files/attachments/2018-08/highAlert2018-Acute-Final.pdf. Accessed 17 April 2020
Classen DC, Pestonik SL, Evans RS, Lloyd JF, Burke JP (1997) Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 277:301–306
Weingart SN, Zhang L, Sweeney M, Hassett M (2018) Chemotherapy medication errors. Lancet Oncol 19:e191–e199
Schwappach DL, Wernli M (2010) Medication errors in chemotherapy: incidence, types and involvement of patients in prevention. A review of the literature. Eur J Cancer Care (Engl) 19:285–292
Ashokkumar R, Srinivasamurthy S, Kelly JJ, Howard SC, Parasuraman S, Uppugunduri CRS (2018) Frequency of chemotherapy medication errors: a systematic review. J Pharmacol Pharmacother 9:86–91
Vélez-Díaz-Pallarés M, Pérez-Menéndez-Conde C, Bermejo-Vicedo T (2018) Systematic review of computerized prescriber order entry and clinical decision support. Am J Health Syst Pharm 75:1909–1921
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Ghaleb MA, Barber N, Franklin BD, Yeung VW, Khaki ZF, Wong IC (2006) Systematic review of medication errors in pediatric patients. Ann Pharmacother 40:1766–1776
Alsulami Z, Conroy S, Choonara I (2013) Medication errors in the Middle East countries: a systematic review of the literature. Eur J Clin Pharmacol 69:995–1008
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 22:153–160
R project (2020) The R project for statistical computing. Available at: http://www.r-project.org. Accessed 17 April 2020
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Aita M, Belvedere O, De Carlo E, Deroma L, De Pauli F, Gurrieri L et al (2013) Chemotherapy prescribing errors: an observational study on the role of information technology and computerized physician order entry systems. BMC Health Serv Res 13:522
Wetterneck TB, Walker JM, Blosky MA, Cartmill RS, Hoonakker P, Johnson MA et al (2011) Factors contributing to an increase in duplicate medication order errors after CPOE implementation. J Am Med Inform Assoc 18:774–782
Chen AR, Lehmann CU (2011) Computerized provider order entry in pediatric oncology: design, implementation, and outcomes. J Oncol Pract 7:218–222
Martin DB, Kaemingk D, Frieze D, Hendrie P, Payne TH (2015) Safe implementation of computerized provider order entry for adult oncology. Appl Clin Inform 6:638–649
Huertas Fernández MJ, Baena-Cañada JM, Martínez Bautista MJ, Arriola Arellano E, García Palacios MV (2006) Impact of computerised chemotherapy prescriptions on the prevention of medication errors. Clin Transl Oncol 8:821–825
Mattsson TO, Holm B, Michelsen H, Knudsen JL, Brixen K, Herrstedt J (2015) Non-intercepted dose errors in prescribing anti-neoplastic treatment: a prospective, comparative cohort study. Ann Oncol 26:981–986
Small MD, Barrett A, Price GM (2008) The impact of computerized prescribing on error rate in a department of Oncology/Hematology. J Oncol Pharm Pract 14:181–187
Khajouei R, Peek N, Wierenga PC, Kersten MJ, Jaspers MW (2010) Effect of predefined order sets and usability problems on efficiency of computerized medication ordering. Int J Med Inform 79:690–698
Kim GR, Chen AR, Arceci RJ, Mitchell SH, Kokoszka KM, Daniel D et al (2006) Error reduction in pediatric chemotherapy: computerized order entry and failure modes and effects analysis. Arch Pediatr Adolesc Med 160:495–498
Voeffray M, Pannatier A, Stupp R, Fucina N, Leyvraz S, Wasserfallen JB (2006) Effect of computerisation on the quality and safety of chemotherapy prescription. Qual Saf Health Care 15:418–421
Markert A, Thierry V, Kleber M, Behrens M, Engelhardt M (2009) Chemotherapy safety and severe adverse events in cancer patients: strategies to efficiently avoid chemotherapy errors in in- and outpatient treatment. Int J Cancer 124:722–728
Collins CM, Elsaid KA (2011) Using an enhanced oral chemotherapy computerized provider order entry system to reduce prescribing errors and improve safety. Int J Qual Health Care 23:36–43
Cheng CH, Chou CJ, Wang PC, Lin HY, Kao CL, Su CT (2012) Applying HFMEA to prevent chemotherapy errors. J Med Syst 36:1543–1551
Elsaid K, Truong T, Monckeberg M, McCarthy H, Butera J, Collins C (2013) Impact of electronic chemotherapy order forms on prescribing errors at an urban medical center: results from an interrupted time-series analysis. Int J Qual Health Care 25:656–663
Meisenberg BR, Wright RR, Brady-Copertino CJ (2014) Reduction in chemotherapy order errors with computerized physician order entry. J Oncol Pract 10:e5–s9
Sanchez Cuervo M, Rojo Sanchis A, Pueyo Lopez C, Gomez de Salazar Lopez de Silanes E, Gramage Caro T, Bermejo Vicedo T (2015) The impact of computerized physician order entry system on medical errors with antineoplastic drugs 5 years after its implementation. J Clin Pharm Ther 40:550–554
Aziz MT, Ur-Rehman T, Qureshi S, Bukhari NI (2015) Reduction in chemotherapy order errors with computerised physician order entry and clinical decision support systems. Health Inf Manag 44:13–22
Wang L, Li Y, Lou Y, Zhang G, Chen J, Wang Y et al (2017) Chemotherapy-related risk management toward safe administration of medications: apply failure mode and effects analysis to reduce the incidence of chemotherapy errors. Pak J Pharm Sci 30:713–720
Chung C, Patel S, Lee R, Fu L, Reilly S, Ho T et al (2018) Implementation of an integrated computerized prescriber order-entry system for chemotherapy in a multisite safety-net health system [published correction appears in Am J Health Syst Pharm 2018 15;75:504]. Am J Health Syst Pharm 75:398–406
Dubeshter B, Walsh CJ, Altobelli K, Loughner J, Angel C (2006) Experience with computerized chemotherapy order entry. J Oncol Pract 2:49–52
Roumeliotis N, Sniderman J, Adams-Webber T, Addo N, Anand V, Rochon P et al Effect of electronic prescribing strategies on medication error and harm in hospital: a systematic review and meta-analysis. J Gen Intern Med 34:2210–2223
Prgomet M, Li L, Niazkhani Z, Georgiou A, Westbrook JI (2017) Impact of commercial computerized provider order entry (CPOE) and clinical decision support systems (CDSSs) on medication errors, length of stay, and mortality in intensive care units: a systematic review and meta-analysis. J Am Med Inform Assoc 24:413–422
Joint commission (2017). The joint commission online. Available at: https://www.jointcommission.org/-/media/deprecated-unorganized/imported-assets/tjc/system-folders/joint-commission-online/jc_online_jan_11pdf.pdf?db=web&hash=4B8781492ABA6035C2398F88489ACCDA. Accessed 17 April 2020
Vermeulen KM, van Doormaal JE, Zaal RJ, Mol PG, Lenderink AW, Haaijer-Ruskamp FM et al (2014) Cost-effectiveness of an electronic medication ordering system (CPOE/CDSS) in hospitalized patients. Int J Med Inform 83(572–580):2014
Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI (2020) An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med 3:17
Rahimi R, Kazemi A, Moghaddasi H, Arjmandi Rafsanjani K, Bahoush G (2018) Specifications of computerized provider order entry and clinical decision support systems for cancer patients undergoing chemotherapy: a systematic review. Chemotherapy 63:162–171
Nuckols TK, Smith-Spangler C, Morton SC, Asch SM, Patel VM, Anderson LJ et al (2014) The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev 3:56
Harshberger CA, Harper AJ, Carro GW, Spath WE, Hui WC, Lawton JM et al (2011) Outcomes of computerized physician order entry in an electronic health record after implementation in an outpatient oncology setting. J Oncol Pract 7:233–237
Acknowledgements
We profusely thank the reviewers for their constructive suggestions for revision of the manuscript. We would also like to thank Editage (www.editage.com) for English language editing.
Funding
Open Access funding provided by Université de Genève. The authors have not declared a specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The CANSEARCH Foundation supports CRS UPPUGUNDURI.
Author information
Authors and Affiliations
Contributions
SKS contributed to the study design, literature search, study quality assessment, data extraction, data analysis, and manuscript preparation. RA was responsible for the research question, literature search, data extraction, and study quality assessment. SK contributed to the literature search and manuscript revision. SCH and SCF contributed to the interpretation and manuscript revision. CRSU contributed to study design, study quality assessment, study analysis, manuscript preparation, and revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 163 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Srinivasamurthy, S., Ashokkumar, R., Kodidela, S. et al. Impact of computerised physician order entry (CPOE) on the incidence of chemotherapy-related medication errors: a systematic review. Eur J Clin Pharmacol 77, 1123–1131 (2021). https://doi.org/10.1007/s00228-021-03099-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03099-9