Abstract
Purpose
To evaluate the role of diltiazem on tacrolimus sparing in pediatric primary nephrotic syndrome (PNS) and its relation to CYP3A4, CYP3A5, ABCB1, and SLCO1B3 polymorphisms.
Methods
The PNS children treated with tacrolimus and with steady-state trough concentration (C0) were retrospectively collected. The impacts of diltiazem on tacrolimus dose-adjusted C0 (C0/D), target concentration achievement, and required dose were evaluated. Meanwhile, the relationship between the polymorphisms (including CYP3A4*1G, CYP3A5*3, ABCB1-C3435T, and SCLO1B3) and dose-sparing effect were investigated.
Results
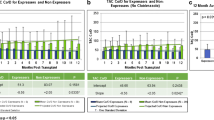
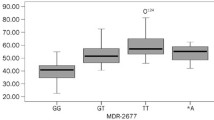
A total of 71 children with 535 concentrations, including 16 children with concomitant diltiazem, were involved. Significantly increased C0/D (94.0 vs 83.8 ng/mL per mg/kg, p = 0.038) and lower required daily dose of tacrolimus (0.056 vs 0.064 mg/kg, p = 0.003) were observed in patients co-administered with diltiazem. Subpopulation carrying CYP3A4*1G, CYP3A5*1, ABCB1-3435TT, or SLCO1B3-699AA was presented with enhanced increment in tacrolimus C0/D by 38.8–102.9%.
Conclusion
Moderate effect of diltiazem on tacrolimus sparing, which might relate to the polymorphisms of CYP3A4, CYP3A5, ABCB1, and SLCO1B3, was documented.


Similar content being viewed by others
Data availability
All data generated during this study are included in this published article.
References
Chadban SJ, Barraclough KA, Campbell SB, Clark CJ, Coates PT, Cohney SJ, Cross NB, Eris JM, Henderson L, Howell MR, Isbel NM, Kanellis J, Kotwal SS, Manley P, Masterson R, Mulley W, Murali K, O'Connell P, Pilmore H, Rogers N, Russ GR, Walker RG, Webster AC, Wiggins KJ, Wong G, Wyburn KR (2012) KHA-CARI guideline: KHA-CARI adaptation of the KDIGO clinical practice guideline for the care of kidney transplant recipients. Nephrology (Carlton) 17(3):204–214. https://doi.org/10.1111/j.1440-1797.2011.01559.x
Lombel RM, Hodson EM, Gipson DS (2013) Treatment of steroid-resistant nephrotic syndrome in children: new guidelines from KDIGO. Pediatr Nephrol 28(3):409–414. https://doi.org/10.1007/s00467-012-2304-8
The Subspecialty Group of Nephrology, Society of Pediatric, Chinese Medical Association (2017) Evidence-based guideline on diagnosis and treatment of steroid-resistant nephrotic syndrome. Chinese J Pediatr 55(11):805–809. https://doi.org/10.3760/cma.j.issn.0578-1310.2017.11.002
Yang EM, Lee ST, Choi HJ, Cho HY, Lee JH, Kang HG, Park YS, Cheong HI, Ha IS (2016) Tacrolimus for children with refractory nephrotic syndrome: a one-year prospective, multicenter, and open-label study of Tacrobell(R), a generic formula. World J Pediatr 12(1):60–65. https://doi.org/10.1007/s12519-015-0062-y
Mangray M, Vella JP (2011) Hypertension after kidney transplant. Am J Kidney Dis 57(2):331–341. https://doi.org/10.1053/j.ajkd.2010.10.048
Bleck JS, Thiesemann C, Kliem V, Christians U, Hecker H, Repp H, Frei U, Westhoff-Bleck M, Manns M, Sewing KF (1996) Diltiazem increases blood concentrations of cyclized cyclosporine metabolites resulting in different cyclosporine metabolite patterns in stable male and female renal allograft recipients. Br J Clin Pharmacol 41(6):551–556. https://doi.org/10.1046/j.1365-2125.1996.34412.x
Mezzano S, Flores C, Ardiles L, Foradori A, Elberg A (1998) Study of neoral kinetics in adult renal transplantation treated with diltiazem. Transplant Proc 30(5):1660–1662. https://doi.org/10.1016/s0041-1345(98)00381-9
Choong CL, Wong HS, Lee FY, Lee CK, Kho JV, Lai YX, Vikneswaran T (2018) Dose-response relationship between diltiazem and tacrolimus and its safety in renal transplant recipients. Transplant Proc 50(8):2515–2520. https://doi.org/10.1016/j.transproceed.2018.04.024
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9(Suppl 3):S1–S157. https://doi.org/10.1111/j.1600-6143.2009.02834.x
Sun JY, Hu YH, Guo HL, Jing X, Xu ZJ, Sun F, Guo HL, Ding XS, Chen F, Xu J (2019) Diltiazem used as a tacrolimus-sparing agent for treatment of pediatric patients with refractory nephrotic syndrome: a case report and retrospective analysis. Eur J Clin Pharmacol 75(4):591–593. https://doi.org/10.1007/s00228-018-2604-4
Jones TE, Morris RG (2002) Pharmacokinetic interaction between tacrolimus and diltiazem: dose-response relationship in kidney and liver transplant recipients. Clin Pharmacokinet 41(5):381–388. https://doi.org/10.2165/00003088-200241050-00005
Kothari J, Nash M, Zaltzman J, Ramesh Prasad GV (2004) Diltiazem use in tacrolimus-treated renal transplant recipients. J Clin Pharm Ther 29(5):425–430. https://doi.org/10.1111/j.1365-2710.2004.00578.x
Staatz CE, Tett SE (2004) Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 43(10):623–653. https://doi.org/10.2165/00003088-200443100-00001
Huang L, Liu Y, Jiao Z, Wang J, Fang L, Mao J (2020) Population pharmacokinetic study of tacrolimus in pediatric patients with primary nephrotic syndrome: a comparison of linear and nonlinear Michaelis-Menten pharmacokinetic model. Eur J Pharm Sci 143:105199. https://doi.org/10.1016/j.ejps.2019.105199
Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G, Starzl T (1995) Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 29(6):404–430. https://doi.org/10.2165/00003088-199529060-00003
Barbarino JM, Staatz CE, Venkataramanan R, Klein TE, Altman RB (2013) PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics 23(10):563–585. https://doi.org/10.1097/FPC.0b013e328364db84
Huang L, Wang J, Yang J, Zhang H, Ni Y, Zhu Z, Wang H, Gao P, Wu Y, Mao J, Fang L (2019) Impact of CYP3A4/5 and ABCB1 polymorphisms on tacrolimus exposure and response in pediatric primary nephrotic syndrome. Pharmacogenomics 20(15):1071–1083. https://doi.org/10.2217/pgs-2019-0090
Zhou S, Yung Chan S, Cher Goh B, Chan E, Duan W, Huang M, McLeod HL (2005) Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet 44(3):279–304. https://doi.org/10.2165/00003088-200544030-00005
Ma Q, Lu AY (2011) Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacol Rev 63(2):437–459. https://doi.org/10.1124/pr.110.003533
Yoshida K, Maeda K, Sugiyama Y (2013) Hepatic and intestinal drug transporters: prediction of pharmacokinetic effects caused by drug-drug interactions and genetic polymorphisms. Annu Rev Pharmacol Toxicol 53:581–612. https://doi.org/10.1146/annurev-pharmtox-011112-140309
Jahan A, Prabha R, Chaturvedi S, Mathew B, Fleming D, Agarwal I (2015) Clinical efficacy and pharmacokinetics of tacrolimus in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 30(11):1961–1967. https://doi.org/10.1007/s00467-015-3133-3
Atanda AT (2012) Chapter 3: Steroid-sensitive nephrotic syndrome in children. Kidney Int Suppl (2011) 2(2):163–171. https://doi.org/10.1038/kisup.2012.16
Cai LL, Huang WQ, Su ZY, Ye HM, Wang LS, Wu Y, Zhang ZY, Zhang W, Tzeng CM (2017) Identification of two novel genes SLC15A2 and SLCO1B3 associated with maintenance dose variability of warfarin in a Chinese population. Sci Rep 7(1):17379. https://doi.org/10.1038/s41598-017-17731-1
Li JL, Wang XD, Chen SY, Liu LS, Fu Q, Chen X, Teng LC, Wang CX, Huang M (2011) Effects of diltiazem on pharmacokinetics of tacrolimus in relation to CYP3A5 genotype status in renal recipients: from retrospective to prospective. Pharmacogenomics J 11(4):300–306. https://doi.org/10.1038/tpj.2010.42
Jones DR, Gorski JC, Hamman MA, Mayhew BS, Rider S, Hall SD (1999) Diltiazem inhibition of cytochrome P-450 3A activity is due to metabolite intermediate complex formation. J Pharmacol Exp Ther 290(3):1116–1125
Yamaori S, Yamazaki H, Iwano S, Kiyotani K, Matsumura K, Honda G, Nakagawa K, Ishizaki T, Kamataki T (2004) CYP3A5 Contributes significantly to CYP3A-mediated drug oxidations in liver microsomes from Japanese subjects. Drug Metab Pharmacokinet 19(2):120–129. https://doi.org/10.2133/dmpk.19.120
Wang WL, Jin J, Zheng SS, Wu LH, Liang TB, Yu SF, Yan S (2006) Tacrolimus dose requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transpl 12(5):775–780. https://doi.org/10.1002/lt.20709
Sakaeda T, Nakamura T, Okumura K (2003) Pharmacogenetics of MDR1 and its impact on the pharmacokinetics and pharmacodynamics of drugs. Pharmacogenomics 4(4):397–410. https://doi.org/10.1517/phgs.4.4.397.22747
Litman T, Druley TE, Stein WD, Bates SE (2001) From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci 58(7):931–959. https://doi.org/10.1007/pl00000912
Kalliokoski A, Niemi M (2009) Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 158(3):693–705. https://doi.org/10.1111/j.1476-5381.2009.00430.x
Tron C, Lemaitre F, Verstuyft C, Petitcollin A, Verdier MC, Bellissant E (2019) Pharmacogenetics of membrane transporters of tacrolimus in solid organ transplantation. Clin Pharmacokinet 58(5):593–613. https://doi.org/10.1007/s40262-018-0717-7
Shen H, Christopher L, Lai Y, Gong J, Kandoussi H, Garonzik S, Perera V, Garimella T, Humphreys WG (2018) Further studies to support the use of coproporphyrin I and III as novel clinical biomarkers for evaluating the potential for organic anion transporting polypeptide 1B1 and OATP1B3 inhibition. Drug Metab Dispos 46(8):1075–1082. https://doi.org/10.1124/dmd.118.081125
Brunet M, van Gelder T, Asberg A, Haufroid V, Hesselink DA, Langman L, Lemaitre F, Marquet P, Seger C, Shipkova M, Vinks A, Wallemacq P, Wieland E, Woillard JB, Barten MJ, Budde K, Colom H, Dieterlen MT, Elens L, Johnson-Davis KL, Kunicki PK, MacPhee I, Masuda S, Mathew BS, Millan O, Mizuno T, Moes DAR, Monchaud C, Noceti O, Pawinski T, Picard N, van Schaik R, Sommerer C, Vethe NT, de Winter B, Christians U, Bergan S (2019) Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit 41(3):261–307. https://doi.org/10.1097/ftd.0000000000000640
Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, Thummel KE (2006) Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug metabolism and disposition: the biological fate of chemicals 34(5):836–847. https://doi.org/10.1124/dmd.105.008680
Funding
The study was funded by the National Natural Science Foundation of China (81773819, 81973396), Natural Science Foundation of Zhejiang Province (Y16H160129, and LSY19H300001), and Zhejiang Provincial Program for 151 Talents (L. Fang).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design; P Gao and J Yang collected the data; Y Hu and H Zhang measured the tacrolimus concentrations and genotyped the SNPs; J Wang and L Fang carried out the data analyses; L Zhang, L Huang, and L Fang contributed to the interpretation of the data; J Wang and L Huang wrote the manuscript; Y Ni and Z Zhu contributed to the revising of the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
The study was performed according to the Declaration of Helsinki and good clinical practice guidelines and approved by the Ethics Committee of the Children’s Hospital, School of Medicine, Zhejiang University.
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Informed consent was obtained from the parents of participants included in the study.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Huang, L., Gao, P. et al. Diltiazem on tacrolimus exposure and dose sparing in Chinese pediatric primary nephrotic syndrome: impact of CYP3A4, CYP3A5, ABCB1, and SLCO1B3 polymorphisms. Eur J Clin Pharmacol 77, 71–77 (2021). https://doi.org/10.1007/s00228-020-02977-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02977-y