Abstract
Background
Spontaneous reporting of adverse drug reactions (ADRs) is an important source of information for post-marketing drug safety evaluation. Most countries have public access to reporting systems, but patients report only 3% of all ADRs. Little is known about factors affecting patient reporting. Our aim was to explore patients’ experiences reporting ADRs and their views on the usability of the Canadian Vigilance reporting forms on MedEffect.
Methods
An interpretive description qualitative study was used. Adults in Canada, who experienced an ADR, were invited to participate through social media (Kijiji, Facebook, Twitter) and by associations (e.g., Patients Canada or Canadian Arthritis Society). Participants were interviewed in English and French using structured interview guides. Inductive content analysis was used.
Results
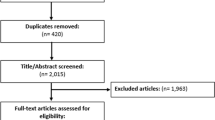
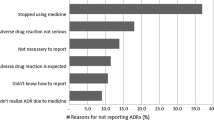
Fifteen interviews were conducted from October 2014 to May 2015. Two participants reported ADRs to MedEffect, and others to physicians and/or pharmacists. Motives for reporting were intolerable side effect impacting daily activities and encouragement from others to report (e.g., family, colleagues). Factors that interfered with reporting were physicians normalized or minimized the side effect, confusion on what to report, no feedback after report submission to MedEffect, and previous experience with side effects. MedEffect forms were described as comprehensive and important, but its usability was affected by the number of questions and complexity of some questions.
Conclusions
Most participants were unaware of MedEffect and reported ADRs to physicians and pharmacists. Several barriers and motives affected patients’ reporting of ADRs. MedEffect form could be simplified for use by patients.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article.
References
Oshikoya KA, Awobusuyi JO (2009) Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. Br J Clin Pharmacol 9:14
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R (1995) Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 274(1):29–34
Blenkinsopp A, Wilkie P, Wang M, Routledge PA (2007) Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol 63(2):148–156
Avery A, Anderson C, Bond C, Fortnum H, Gifford A, Hannaford P et al (2011) Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow Card Scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess 15(20):1–234
Mitchell AS, Henry DA, Sanson-Fisher R, O'Connell DL (1988) Patients as a direct source of information on adverse drug reactions. BMJ 297(6653):891–893
Hazell L, Cornelius V, Hannaford P, Shakir S, Avery AJ (2013) How do patients contribute to signal detection?: A retrospective analysis of spontaneous reporting of adverse drug reactions in the UK’s Yellow Card Scheme. Drug Saf 36(3):199–206. https://doi.org/10.1007/s40264-013-0021-2
van Grootheest K, de Graaf L, de Jong-van den Berg LT (2003) Consumer adverse drug reaction reporting: a new step in pharmacovigilance? Drug Saf 26(4):211–217
Aagaard L, Nielsen LH, Hansen EH (2009) Consumer reporting of adverse drug reactions: a retrospective analysis of the Danish adverse drug reaction database from 2004 to 2006. Drug Saf 32(11):1067–1074
Herxheimer A, Crombag R, Alves TL (2010) Direct patient reporting of adverse drug reactions: a 12-country survey. Briefing Paper[Europe] Health Action International
Hammond IW, Rich DS, Gibbs TG (2007) Effect of consumer reporting on signal detection: using disproportionality analysis. Expert Opin Drug Saf 6(6):705–712
Nichols V, Thériault-Dubé I, Touzin MJ, Delisle JF, Lebel D, Bussières JF et al (2009) Risk perception and reasons for noncompliance in pharmacovigilance. Drug Saf 32(7):579–590
Bäckström M, Mjörndal T, Dahlqvist R, Nordkvist-Olsson T (2000) Attitudes to reporting adverse drug reactions in northern Sweden. Eur J Clin Pharmacol 56(9–10):729–732
Lopez-Gonzalez E, Herdeiro MT, Figueiras A (2009) Determinants of under-reporting of adverse drug reactions. Drug Saf 32(1):19–31
Biriell C, Edwards IR (1997) Reasons for reporting adverse drug reactions—some thoughts based on an international review. Pharmacoepidem Drug Saf 6(1):21–26
Belton KJ, Lewis SC, Payne S, Rawlins MD, Wood SM (1995) Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom [see comments]. Br J Clin Pharmacol 39(3):223–226
Hasford J, Goettler M, Munter KH, Müller-Oerlinghausen B (2002) Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol 55(9):945–950
Herdeiro MT, Figueiras A, Polónia J, Gestal-Otero JJ (2006) Influence of pharmacists’ attitudes on adverse drug reaction reporting. Drug Saf 29(4):331–340
Belton KJ, European pharmacovigilance Research G (1997) Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. Eur J Clin Pharmacol 52(6):423–427
Ekman E, Bäckström M (2009) Attitudes among hospital physicians to the reporting of adverse drug reactions in Sweden. Eur J Clin Pharmacol 65(1):43–46
Herdeiro MT, Figueiras A, Polónia J, Gestal-Otero JJ (2005) Physicians’ attitudes and adverse drug reaction reporting. Drug Saf 28(9):825–833
Grootheest V (1999) Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol 48(4):623–627
Granas AG, Buajordet M, Stenberg-Nilsen H, Harg P, Horn AM (2007) Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidem Drug Saf 16(4):429–434
Passier A, ten Napel M, van Grootheest K, van Puijenbroek E (2009) Reporting of adverse drug reactions by general practitioners. Drug Saf 32(10):851–858
Mes K, Berg LT, Grootheest AC (2002) Attitudes of community pharmacists in the Netherlands towards adverse drug reaction reporting. Int J Pharm Pract 10(4):267–272
Krska J, Jones L, McKinney J, Wilson C (2011) Medicine safety: experiences and perceptions of the general public in Liverpool. Pharmacoepidem Drug Saf 20(10):1098–1103
van Hunsel F, van der Welle C, Passier A, van Puijenbroek E, van Grootheest K (2010) Motives for reporting adverse drug reactions by patient-reporters in the Netherlands. Eur J Clin Pharmacol 66(11):1143–1150
Thorne S (2008) Interpretive description. Left Coast Pr, Walnut Creek
Health Canada. Canada Vigilance Program - Collecting and Assessing Adverse Reaction Reports 2011 [updated 2011-04-28; cited 2014 Jan, 24]. Available from: http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/pubs/medeff/fs-if/2011-cvp-pcv/2011-cvp-pcv-eng.pdf
Creswell JW (2007) Qualitative enquiry and research design: choosing among five approaches. (2nd ed). Sage Publications, Thousand Oaks
Francis JJ, Johnston M, Robertson C, Glidewell L, Entwistle V, Eccles MP, Grimshaw JM (2010) What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 25(10):1229–1245
Denzin NK, Lincoln YS, Giardina MD (2006) Disciplining qualitative research 1. Int J Qual Stud Educ 19(6):769–782
Shenton AK (2004) Strategies for ensuring trustworthiness in qualitative research projects. Educ Inf 22(2):63–75
Lincoln YS, Guba EG (1985) Naturalistic inquiry. Sage, Newbury Park
Lorimer S, Cox A, Langford NJ (2012) A patient’s perspective: the impact of adverse drug reactions on patients and their views on reporting. J Clin Pharm Ther 37(2):148–152
Perry BA, Turner LW (2001) A prediction model for polypharmacy: are older, educated women more susceptible to an adverse drug event? J Women Aging 13(4):39–51
Zopf Y, Rabe C, Neubert A, Gassmann KG, Rascher W, Hahn EG, Brune K, Dormann H (2008 Oct 1) Women encounter ADRs more often than do men. Eur J Clin Pharmacol 64(10):999–1004
van Grootheest K, de Jong-van den Berg L (2004) Patients’ role in reporting adverse drug reactions. Expert Opin Drug Saf 3(4):363–368
Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, Turner MA, Young B (2013) What can we learn from parents about enhancing participation in pharmacovigilance? Br J Clin Pharmacol 75(4):1109–1117
Anderson C, Gifford A, Avery A, Fortnum H, Murphy E, Krska J, Bond C (2012) Assessing the usability of methods of public reporting of adverse drug reactions to the UK Yellow Card Scheme. Health Expect 15(4):433–440
Inch J, Watson MC, Anakwe-Umeh S (2012) Patient versus healthcare professional spontaneous adverse drug reaction reporting: a systematic review. Drug Saf 35(10):807–818
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the University of Ottawa Research Ethics Board in July 2014 (file no. H05-14-18). To ensure confidentiality, all personal identifiers were removed from transcripts and numbers were used to identify participants (e.g., P1, P2…P15). Written informed consent was obtained from all participants prior to their interview.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Dweik, R., Yaya, S., Stacey, D. et al. Patients’ experiences on adverse drug reactions reporting: a qualitative study. Eur J Clin Pharmacol 76, 1723–1730 (2020). https://doi.org/10.1007/s00228-020-02958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02958-1