Abstract
Purpose
To assess the evidence for decision making, at the health care and the patient levels, regarding the use of gene expression assays to inform chemotherapy decisions in breast cancer patients with intermediate clinical risk of recurrence.
Methods
Systematic literature searches were performed (January 2002–April 2020) in Medline, Embase, PubMed, Cochrane Library, PsycINFO and HTA databases. Inclusion criteria: patients (P) were individuals with post-surgical breast cancer at intermediate clinical risk of recurrence; intervention (I)/comparison (C) was (i) use of, versus no use of, a gene expression assay and (ii) withholding versus providing chemotherapy; outcomes (O) were overall survival (OS), health-related quality of life (HRQL), and recurrence. Randomised controlled trials (RCTs) and non-RCTs were included. Random-effects meta-analyses were performed where possible.
Results
Three inconclusive non-RCTs, respectively, compared OS and recurrence with and without a gene expression assay. No studies investigated HRQL. Regarding the comparison withholding versus providing chemotherapy based on a gene expression assay, one RCT and four non-RCTs evaluated OS. In the RCT, 93.9% (I) versus 93.8% (C) were alive at 9 years. Three RCTs and seven non-RCTs evaluated recurrence. Three RCTs could be pooled regarding distant recurrence; 4.29% versus 3.88% had such an event (risk ratio: 1.12 (95% confidence interval: 0.90 to 1.39).
Conclusion
Regarding the use of gene expression assays in breast cancer, evidence on patient effects, informing patient-level chemotherapy decision making, is available. However, evidence for prioritisation at the overall health care level, i.e. use of, versus no use of, such assays, is largely lacking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Personalised medicine is a twenty-first century focus. The concept implies that the drug/treatment choice for a specific patient is based on their biomarker profile. Oncology research has made important contributions within the field; precision cancer medicine aims at providing anti-cancer drugs to those who are likely to respond to the treatment and to avoid such drugs when the opposite can be expected. Indeed, these drugs are often associated with severe adverse reactions which need to be avoided if not clearly counterbalanced by beneficial effects. Furthermore, there may be great heterogeneity between tumours, and treatment may be effective only in a subset of patients. Drug development in oncology has therefore focused on defining genetic and molecular characteristics of the tumour to select patients likely to benefit from treatment [1,2,3,4,5].
Breast cancer, the most common cancer in women and the leading cause of cancer deaths worldwide [6, 7], has been a pioneer target for personalised medicine. The discovery of the oestrogen receptor (ER) and human epidermal growth factor receptor-2 (HER2) has enabled development of blocking therapies. In 2000–2001, the first gene expression profiling data were published, distinguishing subclasses with differences in biology and outcome [8, 9]. Subsequently, gene expression assays were developed to provide prognostic and predictive information to inform chemotherapy decision making. Several assays, covering various tumour genes, are commercially available providing information on the risk of recurrence [10]. To determine the use of this technology in health care, the benefits and risks have to be assessed. Ideally, such assessments should be based on evidence of effects on patients.
About 75% of breast cancers is hormone-sensitive (luminal) and HER2-negative [11]. Treatment decisions are based on the risk of recurrence, determined by tumour stage, and histopathological data and biomarker status as well as menopausal status [12]. For patients at intermediate risk of recurrence, decision making may be particularly difficult; there may be beneficial effects of adjuvant chemotherapy but the risks associated with such treatment are not negligible. Indeed, chemotherapy is associated with fatalities and severe, sometimes persistent, adverse reactions such as neuropathy [13,14,15,16]. Gene expression assays are included in guidelines to identify patients from whom chemotherapy can be withheld [17,18,19]. As far as we are aware, a summarised evidence base is currently lacking regarding patient effects of use of gene expression assays to inform chemotherapy decisions in the subgroup of patients where the clinical risk of recurrence does not suffice for clear-cut decisions. Indeed, previous systematic reviews within the field have had a wider scope [20,21,22,23]. Therefore, we performed this study to assess the evidence on critical patient effects, such as overall survival, recurrence and health-related quality of life (HRQL), of using molecular profiling to inform chemotherapy decisions in this clinically relevant patient group. This evidence is relevant in decision making at both the patient and the health care levels.
Methods
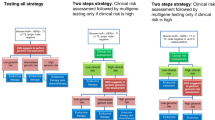
We performed a systematic review according to established routines at the regional health technology assessment (HTA) centre (HTA-centrum) in Region Västra Götaland, Sweden. The aim was defined in two PICOs (Patients, Intervention, Comparison, Outcome; Fig. 1).
Patients and comparison groups for whom the outcomes overall survival, health-related quality of life (HRQL) and recurrence were evaluated. CT, chemotherapy; GEP, gene expression profile; HR, hormone receptor; HER2, human epidermal growth factor 2; N0-1, with no (N0) or one to three (N1) axillary lymph node metastases
In the first PICO, the evidence for horizontal prioritisations was reflected i.e. the scientific basis for decision making regarding the use of gene expression assays in early breast cancer from an overall health care perspective. Patients (P) were individuals with post-surgical breast cancer at intermediate clinical risk of recurrence i.e. ER-positive, HER2-negative and with up to three axillary lymph node metastases (N0-1). The intervention (I) was a gene expression assay, including the patient management and chemotherapy decision making based on the test results. The comparison (C) was no gene expression assay, including standard patient management and chemotherapy decision making. Outcomes (O) were overall survival, HRQL and recurrence. The outcome HRQL was chosen to capture the experience of adverse effects of chemotherapy.
In the second PICO, evidence for decision making at the patient level, i.e. the scientific basis to be guided in chemotherapy decisions by a gene expression assay, was reflected. The patients were the same as those in the first PICO, namely patients in whom the clinical risk of recurrence did not suffice for clear-cut decisions, with the addition of the tumour being categorised as low/intermediate risk of recurrence based on a gene expression assay. The intervention was to withhold chemotherapy, and the comparison was to provide chemotherapy. The outcomes were the same as for the first PICO: overall survival, HRQL and recurrence.
We included both randomised controlled trials (RCTs) and non-randomised controlled trials (non-RCTs). We restricted the search to English or Scandinavian-language (Swedish, Danish and Norwegian) publications.
Literature search and study selection
Systematic searches during August 2018, with updates in January 2019 and April 2020, covering the period from January 2002, were performed in Medline, Embase, PubMed, the Cochrane Library, PsycINFO and a number of HTA databases. Search strategies are provided in Appendix 1. Reference lists of relevant articles were scrutinised for additional references. To identify ongoing or completed but not yet published studies, we searched Clinicaltrials.gov in December 2018, with an update in April 2020.
Identified abstracts were screened by two persons and those that did not meet the PICO criteria were excluded in a consensus discussion. When there were uncertainties regarding inclusion/exclusion, the full text was retrieved. For articles excluded in consensus, after full-text reading, reasons for exclusions were recorded. The remaining studies were included in the systematic review.
Data extraction and quality assessment
Data were extracted from the studies by one author and were subsequently checked by the other authors. Data extraction included the number of individuals in the intervention and control groups, the type of gene expression assay used and the results. When the number of events in the randomisation groups was not available in the original RCT for poolable results, the corresponding author was contacted to obtain the relevant information.
The studies were critically appraised by all authors, according to checklists from the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) [24]. These include assessment of three domains: directness, risk of bias and precision. The authors discussed the assessments and categorised each study as having no or minor problems (+), some problems (?) or major problems (–) in each domain. Disagreements were resolved by discussion. The certainty of evidence, i.e. the confidence in the effect estimate, was then assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [25].
Statistics
RCTs were pooled in random-effects meta-analyses using the software Review Manager (RevMan) version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Heterogeneity was assessed with I2. The individual studies and the pooled estimates were presented in forest plots. Results are presented as risk ratios (RRs) and 95% confidence intervals (CIs).
Results
After removal of duplicates, 2,824 references were identified, 17 of which fulfilled the criteria of either of the PICOs (Fig. 2). Studies excluded after full-text reading are presented in Appendix 2.
Study characteristics
Three RCTs and 14 non-RCTs were included in the review (Table 1). Four non-RCTs investigated patient outcome with and without a gene expression assay [26,27,28,29], while the remaining three RCTs [30,31,32] and ten non-RCTs [33,34,35,36,37,38,39,40,41,42] investigated withholding versus providing chemotherapy. From two RCTs, only a subset of the patients fulfilled the P criteria of this review [30, 31].
Gene expression assay versus no gene expression assay
No RCT and four non-RCTs [26,27,28,29] reported results regarding overall survival and/or recurrence in patients with intermediate clinical risk of recurrence where a gene expression assay had been, versus had not been, performed. No studies evaluated effects on HRQL.
Overall survival
Three studies reported results regarding overall survival, using a cohort design [27, 29] or a historic control [28], and including a total of 60,286 patients. Results favouring the use of a gene expression assay were reported in one study [29], whereas the other two studies reported no difference [27, 28]. The certainty of evidence was downgraded one step as the characteristics of the compared groups either differed [27, 28] or were not reported [29]. In summary, it is uncertain whether use of, versus no use of, a gene expression assay affects overall survival in breast cancer patients with intermediate clinical risk of recurrence (very low certainty of evidence, GRADE ⊕◯◯◯).
Recurrence
Three studies reported results regarding recurrence, using a cohort design [26, 27] or a historic control [28], and including a total of 2,756 patients. One study reported prolonged time to recurrence when a gene expression assay had been used [27], whereas the other two studies reported no difference [26, 28]. The certainty of evidence was downgraded one step as the characteristics of the compared groups differed, and there was some uncertainty about the directness and the precision. In summary, it is uncertain whether use of, versus no use of, a gene expression assay affects recurrence in breast cancer patients with intermediate clinical risk of recurrence (very low certainty of evidence, GRADE ⊕◯◯◯).
Withholding versus providing chemotherapy
Three RCTs and ten cohort studies investigated withholding versus providing chemotherapy regarding the overall survival and/or recurrence in patients with intermediate clinical risk of recurrence and low/intermediate genetic risk of recurrence. No studies evaluated effects on HRQL.
Overall survival
Overall survival was reported in one RCT [32] and four cohort studies [33,34,35, 37]. In the RCT, including 6,711 patients, similar overall survival rates were found at 9 years: 93.9% and 93.8% in the intervention and comparison groups, respectively [32]. Loss to follow-up differed between the comparison groups and was large in relation to the number of events. Three cohort studies reported non-significant results [33, 34, 37], whereas the remaining study reported significantly better outcome for patients who had been administered chemotherapy [35]. In summary, withholding adjuvant chemotherapy to breast cancer patients with intermediate clinical risk of recurrence and low/intermediate risk according to a gene expression assay, compared with providing chemotherapy, probably results in little or no difference in medium-term survival (moderate certainty of evidence, GRADE ⊕⊕⊕◯).
Recurrence
Three RCTs and seven cohort studies reported data on recurrence. The RCT results regarding distant recurrence could be pooled in a meta-analysis; 4.29% and 3.88% of the patients had such an event when chemotherapy was not offered and offered, respectively, based on the results of a gene expression assay. The absolute risk difference was 0.41 percentage points (95% CI: − 0.54 to 1.36). The RR for a distant recurrence was 1.12 (95% CI: 0.90 to 1.39; I2 = 0) (Fig. 3).
Five out of seven cohort studies reported the number of distant recurrences in the comparison groups [36, 39,40,41,42]. In four of these, numerically more patients who had been provided chemotherapy had such an event [36, 39, 41, 42]. The remaining two studies reported either a non-significant hazard ratio with a wide CI [33] or overlapping CIs between the 10-year risk of recurrence of the comparison groups [38]. In three out of the seven cohort studies, patients in the control group were younger and had more advanced cancer [33, 36, 41]. The remaining four studies did not present characteristics of the compared groups [38,39,40, 42].
In summary, withholding adjuvant chemotherapy in breast cancer patients with intermediate clinical risk of recurrence and low/intermediate risk based on a gene expression assay, compared with providing chemotherapy, can probably not exclude a small absolute increased risk of recurrence (moderate certainty of evidence, GRADE ⊕⊕⊕◯).
Ongoing studies
Out of 155 ongoing/completed unpublished studies identified in Clinical Trials, one study completed in 2009 fulfilled the PICO criteria comparing use of a gene expression assay versus no gene expression assay and using a retrospective cohort design (NCT00904566). Two studies, estimated to be completed in 2026 and 2031, respectively, may contribute information regarding the PICO comparing withholding versus providing chemotherapy using a prospective cohort design (NCT03904173, NCT03503799).
Discussion
Our review shows that evidence is largely lacking regarding patient effects of use of, versus no use of, a gene expression assay to inform chemotherapy decisions. Such evidence is useful for prioritisation at the overall health care level. However, there is probably little or no difference in medium-term overall survival when chemotherapy is withheld based on a gene expression assay. Nevertheless, it cannot be ruled out that withholding chemotherapy based on such a test implies an increased risk of recurrence, although the absolute risk is low and the absolute risk difference is small. Given this evidence base, some important knowledge gaps still exist with respect to the use of gene expression assays in breast cancer i.e. personalised medicine. These gaps need to be addressed to inform assessments of the benefit-risk balance.
For diagnostic tools sensitivity and specificity may be the primary issue. For molecular profiling in breast cancer, this has been the main research question [22]. However, as precision cancer medicine is emerging, and the upcoming diagnostic tests imply non-negligible costs, one may argue that scientific evaluations regarding patient effects should be designed and performed to provide a scientific basis for prioritisation.
For technologies to inform chemotherapy decisions, it may be particularly important to evaluate potential effects on HRQL. On the one hand, withholding chemotherapy may increase HRQL because of avoided adverse reactions. On the other, increased fear of recurrence may decrease HRQL [43]. Indeed, to introduce a technology which in itself is costly when available evidence is restricted to negative effects may be problematic. It may be argued that the introduction of gene expression assays will reduce the provision of chemotherapy, thereby reducing the costs to justify a potential worse patient outcome. However, chemotherapy decision making studies in the relevant patient group have reported both increased [44,45,46,47] and decreased [48,49,50,51] administration of chemotherapy when gene expression assay results are provided, and none of these studies had a randomised design. Also, from an ethical perspective, withholding an established treatment may be more problematic than introducing a new one. Therefore, this may call for a more solid evidence base. Conversely, new cancer drugs are sometimes approved based on limited evidence regarding patient-relevant effects [52], and not all meet the threshold for a clinically meaningful effect [53].
Diagnostic tests are used in therapy decision making at the patient level. Most breast cancer patients want to have an active or shared role in decision making regarding chemotherapy [54], as also illustrated in the largest RCT included in this review where the recruitment of patients had to be increased by 73% as 12% of the women chose not to adhere to the assigned treatment [32]. Given the results in the present review, it may be surprising that the guidelines update in 2017 [55], but not in 2019 [17], emphasised that node-positive patients should be informed of the potential benefits from chemotherapy. Indeed, an internationally used “objective” test result may have a large impact on chemotherapy decision making, which is illustrated by the fact that several studies have been performed in patients with intermediate clinical risk of recurrence, in which the chemotherapy decision was based solely on the results of the gene expression assay [26, 45, 56]. Conversely, our results suggest that many oncologists and patients take clinical parameters into account, also when the gene expression assay shows a low/intermediate risk of recurrence. In fact, several cohort studies in this review report that patients given chemotherapy, despite a low/intermediate risk of recurrence according to the gene expression assay, were younger and had more advanced disease [33, 36, 41]. As chemotherapy per se is not likely to increase the risk of recurrence, this finding may also explain that a greater number of distant recurrences occurred in those receiving chemotherapy in cohort studies [36, 41, 42].
In patients with intermediate clinical risk of recurrence and low/intermediate risk of recurrence according to a gene expression assay, the difference between withholding and providing chemotherapy was not statistically significant. However, the confidence interval was quite wide, including an up to 39% increased relative risk of a distant recurrence. To facilitate the process of informing the patient and to contribute to informed decision making, the absolute risk estimate provided in this review may be useful. The mean absolute risk increase of 0.41 percentage points regarding distant recurrence would yield in a number needed to treat (NNT) of 244. Furthermore, the upper confidence limit, of particular interest when investigating non-inferiority, was a 1.36 percentage point increase, yielding a minimum NNT of 74. Consequently, at the minimum, 74 breast cancer patients would have to endure adverse reactions from chemotherapy to avoid one distant recurrence.
As patients live many years after a breast cancer diagnosis, as illustrated by the fact that 94% was still alive after 9 years in the main RCT [32], it would take a long time to achieve mature data on long-term survival when a gene expression assay is used to guide treatment decisions. Indeed, the risk of distant recurrence and death from oestrogen dependent breast cancer persists over at least 20 years, also in low-risk patients [57]. Analyses of register data may contribute valuable information in the meantime, in particular as our evidence synthesis shows that an increased risk, although small in absolute numbers, of distant recurrence cannot be excluded if chemotherapy is withheld based on genetic testing. However, as current drug effectiveness and safety studies often have major methodological problems [58, 59], scientific rigour in the design and reporting will be crucial. For example, efforts have to be made to balance the comparison groups with respect to the severity of disease. Indeed, where data on characteristics of the comparison groups were available in the cohort studies in this review, patients administered chemotherapy had more advanced cancer. It is noteworthy that the one study with minor study limitations evaluating overall survival in matched comparisons reported better outcomes for those treated with chemotherapy [35].
Multivariable analysis may provide information on the association between various factors and patient outcome. Unfortunately, none of the studies in this review which performed such analyses included provision of chemotherapy in the analysis [34,35,36,37,38, 40, 41]. Although pharmacoepidemiological studies should ideally be specifically designed to evaluate drug effects [58, 59], inclusion of the provision of chemotherapy would be of interest. Importantly, causality cannot be claimed in such analyses; a cross-sectional design would be applied although seemingly mimicking a cohort design [58].
Strengths and limitations
The main strength of this systematic review and meta-analysis is that it gives an overview of the compiled current evidence on patient effects using gene expression assays in the subgroup of breast cancer patients where the clinical risk of recurrence does not suffice for clear-cut decisions. In addition, the findings are discussed in a wider context, which is of relevance for decision making at both the health care and the patient levels and for future research within personalised medicine. Indeed, precision cancer medicine is a rapidly growing field.
Limitations include that few studies fulfilled our PICO criteria, in particular regarding the comparison of patient effects of use versus no use of a gene expression assay. Furthermore, the CI for the RR in the meta-analysis, comparing withholding versus providing chemotherapy, was fairly wide, ranging from 10% decreased to 39% increased risk of distant recurrence. Translated to absolute numbers, the risk variation was small, from 0.5% decreased risk to 1.4% increased risk. Nevertheless, the as-treated analysis in the largest RCT showed superiority for the primary composite outcome (invasive-disease recurrence, second primary cancer or death) for the randomisation group allocated to chemotherapy, according to the predetermined statistical non-inferiority definitions [32], supporting the conclusion that an increase in recurrence cannot be excluded in those not allocated to chemotherapy.
Conclusion
In summary, this systematic review and meta-analysis illustrates that the evidence base for decision making at the overall health care level regarding the use of a gene expression assay to guide chemotherapy decisions in breast cancer with intermediate risk of recurrence is still limited. For decision making at the patient level, on the other hand, evidence is more solid; withholding chemotherapy based on the results of such a genetic tumour test probably yields similar chances of medium-term survival, but an increased risk of recurrence, though small in absolute numbers, cannot be excluded. As breast cancer research may be considered fairly advanced within the field of personalised medicine, our results may encourage an increased focus in precision cancer medicine to contribute evidence essential for horizontal prioritisation i.e. a scientific basis for assessments of the overall benefit-risk balance.
Data availability
All data analysed during the current study are provided in the article and in the supplement.
References
Doroshow DB, Doroshow JH (2019) From the broad phase II trial to precision oncology: a perspective on the origins of basket and umbrella clinical trial designs in cancer drug development. Cancer J 25(4):245–253. https://doi.org/10.1097/ppo.0000000000000386
Janiaud P, Serghiou S, Ioannidis JPA (2019) New clinical trial designs in the era of precision medicine: an overview of definitions, strengths, weaknesses, and current use in oncology. Cancer Treat Rev 73:20–30. https://doi.org/10.1016/j.ctrv.2018.12.003
Garralda E, Dienstmann R, Piris-Gimenez A, Brana I, Rodon J, Tabernero J (2019) New clinical trial designs in the era of precision medicine. Mol Oncol 13(3):549–557. https://doi.org/10.1002/1878-0261.12465
Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, White C, Lowe C, Sherba JJ, Hartmanshenn C, O'Neill KM, Balter ML, Fritz ZR, Androulakis IP, Schloss RS, Yarmush ML (2018) The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci) 6(3-4):79–100. https://doi.org/10.1142/s2339547818300020
Thompson MA, Godden JJ, Wham D, Ruggeri A, Mullane MP, Wilson A, Virani S, Weissman SM, Ramczyk B, Vanderwall P, Weese JL (2019) Coordinating an oncology precision medicine clinic within an integrated health system: lessons learned in year one. J Patient Cent Res Rev 6(1):36–45. https://doi.org/10.17294/2330-0698.1639
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Carioli G, Malvezzi M, Rodriguez T, Bertuccio P, Negri E, La Vecchia C (2017) Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 36:89–95. https://doi.org/10.1016/j.breast.2017.06.003
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98(19):10869–10874. https://doi.org/10.1073/pnas.191367098
Lal S, McCart Reed AE, de Luca XM, Simpson PT (2017) Molecular signatures in breast cancer. Methods 131:135–146. https://doi.org/10.1016/j.ymeth.2017.06.032
Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR (2005) Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol 123(1):21–27. https://doi.org/10.1309/4wv79n2ghj3x1841
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thurlimann B, Andre F, Baselga J, Bergh J, Bonnefoi H, Brucker SY, Cardoso F, Carey L, Ciruelos E, Cuzick J, Denkert C, Di Leo A, Ejlertsen B, Francis P, Galimberti V, Garber J, Gulluoglu B, Goodwin P, Harbeck N, Hayes DF, Huang CS, Huober J, Hussein K, Jassem J, Jiang Z, Karlsson P, Morrow M, Orecchia R, Osborne KC, Pagani O, Partridge AH, Pritchard K, Ro J, Rutgers EJT, Sedlmayer F, Semiglazov V, Shao Z, Smith I, Toi M, Tutt A, Viale G, Watanabe T, Whelan TJ, Xu B (2017) De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 28(8):1700–1712. https://doi.org/10.1093/annonc/mdx308
Fontes F, Pereira S, Castro-Lopes JM, Lunet N (2016) A prospective study on the neurological complications of breast cancer and its treatment: updated analysis three years after cancer diagnosis. Breast 29:31–38. https://doi.org/10.1016/j.breast.2016.06.013
Rivera DR, Ganz PA, Weyrich MS, Bandos H, Melnikow J (2018) Chemotherapy-associated peripheral neuropathy in patients with early-stage breast cancer: a systematic review. J Natl Cancer Inst 110(2). https://doi.org/10.1093/jnci/djx140
Simon NB, Danso MA, Alberico TA, Basch E, Bennett AV (2017) The prevalence and pattern of chemotherapy-induced peripheral neuropathy among women with breast cancer receiving care in a large community oncology practice. Qual Life Res 26(10):2763–2772. https://doi.org/10.1007/s11136-017-1635-0
Greenwald MK, Ruterbusch JJ, Beebe-Dimmer JL, Simon MS, Albrecht TL, Schwartz AG (2019) Risk of incident claims for chemotherapy-induced peripheral neuropathy among women with breast cancer in a Medicare population. Cancer 125(2):269–277. https://doi.org/10.1002/cncr.31798
Andre F, Ismaila N, Henry NL, Somerfield MR, Bast RC, Barlow W, Collyar DE, Hammond ME, Kuderer NM, Liu MC, Van Poznak C, Wolff AC, Stearns V (2019) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol 37(22):1956–1964. https://doi.org/10.1200/jco.19.00945
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F (2015) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v8–v30. https://doi.org/10.1093/annonc/mdv298
National Institute for Health and Care Excellence (NICE) (2017) Tumour profiling tests to guide adjuvant chemotherapy decisions in people with breast cancer (update of DG10). https://www.niceorguk/guidance/dg34/documents/diagnostics-assessment-report
Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC, Mennel RG, Van Poznak C, Bast RC, Hayes DF (2016) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34(10):1134–1150. https://doi.org/10.1200/jco.2015.65.2289
Chang MC, Souter LH, Kamel-Reid S, Rutherford M, Bedard P, Trudeau M, Hart J, Eisen A (2017) Clinical utility of multigene profiling assays in early-stage breast cancer. Curr Oncol 24(5):e403–e422. https://doi.org/10.3747/co.24.3595
Blok EJ, Bastiaannet E, van den Hout WB, Liefers GJ, Smit V, Kroep JR, van de Velde CJH (2018) Systematic review of the clinical and economic value of gene expression profiles for invasive early breast cancer available in Europe. Cancer Treat Rev 62:74–90. https://doi.org/10.1016/j.ctrv.2017.10.012
(Quality) OH (2020) Gene expression profiling tests for early-stage invasive breast cancer: a health technology assessment. Ont Health Technol Assess Ser 20 (10): 1-234
Swedish Agency for Health Technology Assessment and Assessment of Social Services (2014) Checklist for quality assessment of randomised/observation studies [Mall för kvalitetsgranskning av randomiserade/observationsstudier]. In: ed.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
Rath MG, Uhlmann L, Fiedler M, Heil J, Golatta M, Dinkic C, Hennigs A, Schott S, Ernst V, Koch T, Sohn C, Brucker C, Rom J (2018) Oncotype DX((R)) in breast cancer patients: clinical experience, outcome and follow-up-a case-control study. Archives of Gynecology and Obstetrics 297(2):443–447. https://doi.org/10.1007/s00404-017-4618-z
Pomponio M, Keele L, Hilt E, Burkbauer L, Goldbach M, Nazarian S, Fox K, Tchou J (2020) Impact of 21-gene expression assay on clinical outcomes in node-negative <= T1b breast cancer. Annals of Surgical Oncology 27(5):1671–1678. https://doi.org/10.1245/s10434-019-08028-w
Thibodeau S, Voutsadakis IA (2019) The Oncotype Dx assay in ER-positive, HER2-negative breast cancer patients: a real life experience from a single cancer center. Eur J Breast Health 15(3):163–170. https://doi.org/10.5152/ejbh.2019.4901
Zhang L, Hsieh MC, Petkov V, Yu Q, Chiu YW, Wu XC (2020) Trend and survival benefit of Oncotype DX use among female hormone receptor-positive breast cancer patients in 17 SEER registries, 2004-2015. Breast Cancer Research & Treatment 180(2):491–501. https://doi.org/10.1007/s10549-020-05557-x
Cardoso F, van't Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M, Investigators M (2016) 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375(8):717–729
Geyer CE Jr, Tang G, Mamounas EP, Rastogi P, Paik S, Shak S, Baehner FL, Crager M, Wickerham DL, Costantino JP, Wolmark N (2018) 21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer. NPJ Breast Cancer 4:37. https://doi.org/10.1038/s41523-018-0090-6
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121
Barcenas CH, Raghavendra A, Sinha AK, Syed MP, Hsu L, Patangan MG Jr, Chavez-MacGregor M, Shen Y, Hortobagyi GH, Valero V, Giordano SH, Ueno NT, Tripathy D (2017) Outcomes in patients with early-stage breast cancer who underwent a 21-gene expression assay. Cancer 123(13):2422–2431
Chen J, Wu X, Christos PJ, Formenti S, Nagar H (2018) Practice patterns and outcomes for patients with node-negative hormone receptor-positive breast cancer and intermediate 21-gene Recurrence Scores. Breast Cancer Res 20(1):26
Ibraheem AF, Press DJ, Olopade OI, Huo D (2019) Community clinical practice patterns and mortality in patients with intermediate oncotype DX recurrence scores: who benefits from chemotherapy? Cancer 125(2):213–222. https://doi.org/10.1002/cncr.31818
Le Du F, Gonzalez-Angulo AM, Park M, Liu DD, Hortobagyi GN, Ueno NT (2015) Effect of 21-Gene RT-PCR Assay on adjuvant therapy and outcomes in patients with stage I breast cancer. Clin Breast Cancer 15(6):458–466. https://doi.org/10.1016/j.clbc.2015.06.006
Park S, Han Y, Liu Y, Toriola AT, Peterson LL, Colditz GA, Kim SI, Cho YU, Park BW, Park Y (2019) Adjuvant chemotherapy and survival among patients 70 years of age and younger with node-negative breast cancer and the 21-gene recurrence score of 26-30. Breast Cancer Research 21(1):110. https://doi.org/10.1186/s13058-019-1190-4
Sestak I, Martin M, Dubsky P, Kronenwett R, Rojo F, Cuzick J, Filipits M, Ruiz A, Gradishar W, Soliman H, Schwartzberg L, Buus R, Hlauschek D, Rodriguez-Lescure A, Gnant M (2019) Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast Cancer Research & Treatment 176(2):377–386. https://doi.org/10.1007/s10549-019-05226-8
Stemmer SM, Steiner M, Rizel S, Ben-Baruch N, Uziely B, Jakubowski DM, Baron J, Shak S, Soussan-Gutman L, Bareket-Samish A, Fried G, Rosengarten O, Itay A, Nisenbaum B, Katz D, Leviov M, Tokar M, Liebermann N, Geffen DB (2019) Ten-year clinical outcomes in N0 ER+ breast cancer patients with Recurrence Score-guided therapy. NPJ Breast Cancer 5:41. https://doi.org/10.1038/s41523-019-0137-3
Stemmer SM, Steiner M, Rizel S, Geffen DB, Nisenbaum B, Peretz T, Soussan-Gutman L, Bareket-Samish A, Isaacs K, Rosengarten O, Fried G, McCullough D, Svedman C, Shak S, Liebermann N, Ben-Baruch N (2017b) Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. NPJ Breast Cancer 3:32. https://doi.org/10.1038/s41523-017-0033-7
Stemmer SM, Steiner M, Rizel S, Soussan-Gutman L, Ben-Baruch N, Bareket-Samish A, Geffen DB, Nisenbaum B, Isaacs K, Fried G, Rosengarten O, Uziely B, Svedman C, McCullough D, Maddala T, Klang SH, Zidan J, Ryvo L, Kaufman B, Evron E, Karminsky N, Goldberg H, Shak S, Liebermann N (2017a) Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: evidence from a large prospectively designed registry. NPJ Breast Cancer 3:33
Wen HY, Krystel-Whittemore M, Patil S, Pareja F, Bowser ZL, Dickler MN, Norton L, Morrow M, Hudis CA, Brogi E (2017) Breast carcinoma with an Oncotype Dx recurrence score < 18: rate of distant metastases in a large series with clinical follow-up. Cancer 123(1):131–137. https://doi.org/10.1002/cncr.30271
Koch L, Bertram H, Eberle A, Holleczek B, Schmid-Hopfner S, Waldmann A, Zeissig SR, Brenner H, Arndt V (2014) Fear of recurrence in long-term breast cancer survivors-still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the cancer survivorship--a multi-regional population-based study. Psychooncology 23(5):547–554. https://doi.org/10.1002/pon.3452
Dieci MV, Guarneri V, Giarratano T, Mion M, Tortora G, De Rossi C, Gori S, Oliani C, Merlini L, Pasini F, Bonciarelli G, Griguolo G, Orvieto E, Michieletto S, Saibene T, Del Bianco P, De Salvo GL, Conte P (2018) First prospective multicenter italian study on the impact of the 21-gene recurrence score in adjuvant clinical decisions for patients with er positive/her2 negative breast cancer. Oncologist 23(3):297–305
Martinez Del Prado P, Alvarez-Lopez I, Dominguez-Fernandez S, Plazaola A, Ibarrondo O, Galve-Calvo E, Ancizar-Lizarraga N, Gutierrez-Toribio M, Lahuerta-Martinez A, Mar J (2018) Clinical and economic impact of the 21-gene recurrence score assay in adjuvant therapy decision making in patients with early-stage breast cancer: pooled analysis in 4 Basque Country university hospitals. Clinicoecon Outcomes Res 10:189–199. https://doi.org/10.2147/ceor.s146095
Panousis D, Ntasiou P, Grosomanidis D, Chatzopoulos K, Paraskevakou G, Kontogianni P, Charitidou E, Xepapadakis G (2017) Impact of Oncotype DX on chemotherapy assignment: a retrospective single-center study on female breast cancer patients. Journal of BUOn 22(5):1199–1208
Zhang YN, Zhou YD, Mao F, Sun Q (2015) Impact of the 21-Gene Recurrence Score Assay in adjuvant chemotherapy selection for node-negative, hormone receptor-positive breast cancer in the Chinese population. Neoplasma 62(4):658–665
Friese CR, Li Y, Bondarenko I, Hofer TP, Ward KC, Hamilton AS, Deapen D, Kurian AW, Katz SJ (2017) Chemotherapy decisions and patient experience with the recurrence score assay for early-stage breast cancer. Cancer 123(1):43–51
Kuchel A, Robinson T, Comins C, Shere M, Varughese M, Sparrow G, Sahu A, Saunders L, Bahl A, Cawthorn SJ, Braybrooke JP (2016) The impact of the 21-gene assay on adjuvant treatment decisions in oestrogen receptor-positive early breast cancer: a prospective study. Br J Cancer 114(7):731–736
Schreuder K, Kuijer A, Bentum S, van Dalen T, Siesling S (2018) Use and Impact of the 21-Gene recurrence score in relation to the clinical risk of developing metastases in early breast cancer patients in the Netherlands. Public Health Genomics 21(1-2):1–8. https://doi.org/10.1159/000495742
Wuerstlein R, Sotlar K, Gluz O, Otremba B, von Schumann R, Witzel I, Schindlbeck C, Janni W, Schem C, Bauerfeind I, Hasmueller S, Tesch H, Paulenz A, Ghali N, Orujov E, Kates RE, Cowens W, Hornberger J, Pelz E, Harbeck N (2016) The West German Study Group Breast Cancer Intrinsic Subtype study: a prospective multicenter decision impact study utilizing the Prosigna assay for adjuvant treatment decision-making in estrogen-receptor-positive, HER2-negative early-stage breast cancer. Curr Med Res Opin 32(7):1217–1224. https://doi.org/10.1185/03007995.2016.1166102
Wallerstedt SM, Henriksson M (2018) Balancing early access with uncertainties in evidence for drugs authorized by prospective case series - systematic review of reimbursement decisions. Br J Clin Pharmacol 84(6):1146–1155. https://doi.org/10.1111/bcp.13531
Del Paggio JC, Azariah B, Sullivan R, Hopman WM, James FV, Roshni S, Tannock IF, Booth CM (2017) Do contemporary randomized controlled trials meet ESMO thresholds for meaningful clinical benefit? Ann Oncol 28(1):157–162. https://doi.org/10.1093/annonc/mdw538
Hamelinck VC, Bastiaannet E, Pieterse AH, van de Velde CJH, Liefers GJ, Stiggelbout AM (2018) Preferred and perceived participation of younger and older patients in decision making about treatment for early breast cancer: a prospective study. Clin Breast Cancer 18(2):e245–e253. https://doi.org/10.1016/j.clbc.2017.11.013
Krop I, Ismaila N, Andre F, Bast RC, Barlow W, Collyar DE, Hammond ME, Kuderer NM, Liu MC, Mennel RG, Van Poznak C, Wolff AC, Stearns V (2017) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol 35(24):2838–2847. https://doi.org/10.1200/jco.2017.74.0472
Loncaster J, Armstrong A, Howell S, Wilson G, Welch R, Chittalia A, Valentine WJ, Bundred NJ (2017) Impact of Oncotype DX breast Recurrence Score testing on adjuvant chemotherapy use in early breast cancer: real world experience in Greater Manchester, UK. Eur J Surg Oncol 43(5):931–937
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, Hayes DF (2017) 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377(19):1836–1846. https://doi.org/10.1056/NEJMoa1701830
Wallerstedt SM, Hoffmann M (2019) Evidence synthesis based on non-randomised studies-a critical review of studies leading to conclusions on fall risk properties of loop diuretics/beta-blockers. Eur J Clin Pharmacol 75(12):1731–1738. https://doi.org/10.1007/s00228-019-02754-6
Wallerstedt SM, Hoffmann M (2017) Evaluating beneficial drug effects in a non-interventional setting: a review of effectiveness studies based on Swedish Prescribed Drug Register data. Br J Clin Pharmacol 83(6):1309–1318. https://doi.org/10.1111/bcp.13206
Acknowledgements
Open access funding provided by University of Gothenburg. The authors are grateful to the HTA librarians Ida Stadig and Therese Svanberg who performed the literature search and the initial exclusion of studies according to the inclusion/exclusion criteria.
Author information
Authors and Affiliations
Contributions
B.L. conceived the study, and S.M.W. and A.S designed it. All authors took part in the process of including and excluding articles after the initial exclusion by the librarians. S.M.W. and A.N.E. extracted data from the included studies, which were checked by R.O.B., A.K., A.S. and B.L. All authors assessed the study quality. S.M.W. and A.S. performed the meta-analysis. S.M.W., A.N.E. and B.L. drafted the manuscript and R.O.B., A.K. and A.S. revised it. S.M.W. is the guarantor of the study.
Corresponding author
Ethics declarations
No ethics approval was required as no sensitive data were handled.
Conflict of interest
S.M.W., A.N.E. and A.S. have no conflicts of interest to declare. R.O.B. has received research grants from Astra Zeneca, speaker honoraria from Roche and Pfizer, and has served on advisory boards for Amgen, BMS, Novartis and MSD. A.K. has a consulting and advisory role for Pfizer and Roche and has received honoraria from these companies. B.L. is a member of advisory boards for Pfizer, Astra Zeneca and Eli Lilly.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wallerstedt, S.M., Nilsson Ek, A., Olofsson Bagge, R. et al. Personalised medicine and the decision to withhold chemotherapy in early breast cancer with intermediate risk of recurrence – a systematic review and meta-analysis. Eur J Clin Pharmacol 76, 1199–1211 (2020). https://doi.org/10.1007/s00228-020-02914-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02914-z