Abstract
Purpose
Proton pump inhibitor (PPI) drugs are approved for the management of gastric acid–related diseases, mainly treatment of gastroesophageal reflux disease, treatment of nonsteroidal anti-inflammatory drugs (NSAID)–related gastrointestinal complications and prevention in at-risk patients, Helicobacter pylori eradication, and treatment of ulcers. PPIs are one of the most commonly prescribed drug class worldwide, and off-label use is widespread. The aim of this study was to describe outpatient PPI use of the whole adult population in France, based on the French National Health Data System (SNDS).
Methods
All individuals aged 18 years or older, with at least one dispensing for PPI between January 1, 2015 and December 31, 2015, were identified as PPI users. PPI users were considered as new users if they received no dispensing for PPI in the prior year. New users were followed until treatment discontinuation or up to 1 year, whichever occurred first. Characteristics of new users and of their PPI treatment were described, overall and separately by treatment indication.
Results
In total, 15,388,419 PPI users were identified in 2015 (57.0% women; mean age 57.0 years), accounting for 29.8% of the French adult population. Of them, 7,399,303 were new PPI users; mean treatment duration was 40.9 days, and 4.1% received a continuous PPI therapy lasting more than 6 months (10.2% among new users > 65 years versus 2.4% among those 18–65 years). For 53.5% of new users, indication for PPI therapy was a co-prescription with NSAID; in this indication, the large majority of patients (79.7%) had no measurable risk factor supporting a systematic prophylactic co-prescription of PPI. A proportion of 32.4% of new users did not have any identified comedication or inpatient diagnosis supporting an indication for PPI therapy; among them, only a small proportion (7.3% overall, and 8.4% of patients aged > 65 years) underwent a procedure investigating the digestive tract at the time of PPI initiation.
Conclusion
The results of this study suggest PPI overuse in France, not always in line with the French guidelines. In particular, inappropriate co-prescription with NSAID was frequent. Efforts should be made to limit PPI treatment to appropriate indications and durations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proton pump inhibitors (PPIs) are a class of drugs suppressing gastric acid secretion. Their use has dramatically grown since their market introduction in the late 1980s, and they are currently one of the most commonly prescribed drugs worldwide [1]. In the UK, PPI use more than doubled since 2007, ranking second by prescription items in 2017, with nearly 59 million items dispensed annually [2]. In France, PPI sales increased by 20% between years 2010 and 2013, with 80 million packs in 2013 (source: National Agency for Medicines and Health Products Safety [ANSM]), with esomeprazole, omeprazole, and pantoprazole being among the top 30 best-selling drugs in pharmacies [3]. PPIs are approved in the following main indications [4,5,6,7]: treatment of gastroesophageal reflux disease (GERD) and reflux esophagitis, treatment of nonsteroidal anti-inflammatory drugs (NSAID)–associated gastrointestinal (GI) lesions and prevention in at-risk patients (aged > 65 years, with a history of GI ulcer, or with concomitant antiplatelet, anticoagulant, or corticosteroid therapy), Helicobacter pylori eradication, and treatment of peptic ulcer. Off-label prescribing has been widely reported, including in non-at-risk patients taking antiplatelet, anticoagulant, or corticosteroid drugs without concomitant NSAID, in prevention of chemotherapy or radiotherapy-associated lesions in patients with cancer, or in the management of extra-digestive symptoms potentially from gastroesophageal reflux, including non-cardiac chest pain, laryngeal complaints, asthma, or chronic cough [8, 9]. The estimated rate of inappropriate use reaches 50% [10]. In France, previous studies on PPI use were limited to specific subpopulations or healthcare settings [11,12,13,14,15,16,17,18,19,20,21,22]. The objective of this study was to describe PPI use, and its appropriateness, at the whole French population level, based on data from national medico-administrative databases.
Methods
Datasources
The French National Health Data System (Système National des Données de Santé, SNDS) covers the entire French population (66 million inhabitants) irrespective of employment status. It contains two main databases: the health insurance claims database (DCIR) and the national hospital discharge database (PMSI). An anonymous, unique identifier for each individual links the DCIR information to the PMSI. The DCIR contains individual data on sociodemographic characteristics and all medical claims since 2008. It provides information on dispensed drugs (recorded according to the Anatomical Therapeutic Chemical [ATC] classification system) with date of delivery, therapeutic procedures coded using a common classification of medical procedures (Classification Commune des Actes Médicaux [CCAM]), and laboratory tests. The database also contains medical information on the presence of any serious and costly long-term disease giving entitlement to a 100% health insurance coverage, with information on diagnosis encoded in the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) and date of disease onset. The PMSI includes information on every admission in a public or private hospital in France since 2006, either for an inpatient stay or ambulatory care. Diagnoses (in ICD-10 codes) and medical or surgical procedures provided during hospital stays are available.
Study population
All individuals aged 18 years or older, with at least one dispensing for PPI between January 1, 2015 and December 31, 2015, were identified as PPI users. The first dispensing date for PPI in 2015 was the index date. PPI users were considered as new users if they did not receive any dispensing for PPI in the year prior to the index date. New users were followed from the index date until treatment discontinuation, if any, or up to 1 year.
PPI treatment characteristics
PPIs were identified by the ATC classification A02BC. Treatment duration was estimated by the number of dispensed tablets of PPIs, assuming that one tablet corresponds to 1 day of exposure, except for eradication of H. pylori, requiring double dose. If a patient failed to fill a new prescription within the estimated dispensing duration plus a 30-day grace period, we considered that the treatment was discontinued. PPI users with a treatment lasting more than 6 months were considered as long-term users. Prescribers were categorized as hospital or private practice practitioner. Information on prescriber specialty was available for private practitioners only.
Although the reason for prescription is not explicitly recorded in the SNDS, individual information on concomitant medications, inpatient diagnoses, and medical and laboratory procedures was collected to identify the probable indication for PPI treatment among new users, according to an algorithm detailed in Fig. 1. The sequence of indications was established based on data from the international literature, experts’ opinion, and prescribing recommendations. The algorithm was designed to identify, as much as possible for each patient, an indication in accordance with the French guidelines, if any. This included eradication of H. pylori (defined by both dispensing data suggestive of eradication antibiotic regimens, and inpatient diagnosis of H. pylori infection/or specific laboratory testing [23]), or treatment/prevention of NSAIDs-associated ulcers. We then considered indications potentially not in line with the French guidelines but observed in clinical practice, including co-prescription with antiplatelet or anticoagulant therapy, corticosteroid therapy, or chemotherapy or radiotherapy in patients with cancer. For patients remaining uncategorized, we searched for inpatient diagnosis of digestive lesion. Finally, in the absence of informative data, the indication was considered indeterminate. For the sake of simplicity, every new PPI user was assigned a unique indication. Nevertheless, for each indication, patients’ characteristics regarding concomitant medications, gastro-intestinal procedures, and diagnosis were also described.
Sociodemographic and medical characteristics
For each PPI user, individual information was available on the following: sociodemographic characteristics at the index date, including sex and age; medical characteristics including gastrointestinal procedures (upper gastrointestinal endoscopy, with or without biopsy, upper gastrointestinal series, esophageal manometry, and pH examination) and inpatient diagnoses of gastrointestinal diseases (ICD-10 codes listed in the supplementary materials), recorded within the 12 months preceding the index date and, taking into account possible diagnostic process delays after PPI initiation, in the 6 months following; and comedications defined by drugs dispensed within the 30 days preceding or following the index date.
Statistical analyses
The frequency of PPI use was computed by dividing the total number of PPI users in 2015 by the estimated total French adult population on January 1, 2015 [24]. The proportion of new PPI users was calculated by dividing the number of new PPI users by the total number of PPI users in 2015. Characteristics of new PPI users and of their PPI treatment were described, overall and separately by PPI treatment indication. All analyses were performed with SAS EG® (SAS Institute, Cary, NC, USA), 7.13.
Results
In total, 15,388,419 adults aged 18 years or older had at least one dispensing for PPI in 2015 (57.0% women; mean age 57.0 years). PPI users accounted for 29.8% of the estimated French adult population (15,388,419/51,645,958): 25.1% among the 18–65 years (10,096,055/40,229,778) and 46.4% among those aged > 65 years (5,292,364/11,416,180).
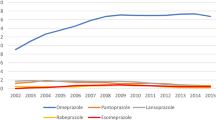
Among all PPI users in 2015, 7,399,303 (48.1%) were new users. Omeprazole was the most commonly dispensed PPI at treatment initiation (44.7% of new users) (Table 1). PPI therapy was initiated by a private practitioner in 73.9% of cases, 87.1% of them being general practitioners and 1.7% hepato-gastroenterologists. A majority of new users (78.0%) had only one dispensing. Mean treatment duration was 40.9 days overall, and was higher among patients aged > 65 years than among those aged 18–65 years (65.0 days versus 34.3 days). Patients among whom PPI therapy was prolonged > 6 months accounted for 4.1% of new users overall. This proportion was higher among patients aged > 65 years than among those aged 18–65 years (10.2% versus 2.4%).
Each new PPI user was assigned a unique indication: H. pylori eradication was identified for 0.5% of patients; co-prescription with NSAID for 53.5%; co-prescription with antiplatelet/anticoagulant therapy for 5.2%; co-prescription with corticosteroid therapy for 5.3%; co-prescription with chemotherapy or radiotherapy in patients with cancer for 0.5%; and treatment of other documented digestive lesions for 2.5%. Indication could not be established for 32.4% of new users. Marked differences were observed between age categories, especially regarding co-prescription with NSAID (43.5% among new users aged > 65 years versus 56.2% among those aged 18–65 years) or with antiplatelet/anticoagulant therapy (15.3% among new users aged > 65 years versus 2.4% among those aged 18–65 years) (Table 1).
Characteristics of new PPI users and of their treatment are described separately by indication in Table 2.
In new PPI users treated for H. pylori eradication (59.1% women; mean age 50.7 years), PPI therapies were most often initiated by private practitioners (in 71.7% of cases; 64.2% of them being general practitioners and 30.3% hepato-gastroenterologists). Mean treatment duration was 24.7 days.
In new PPI users with a co-prescription of NSAID (54.7% women; mean age 49.8 years), PPI treatments were largely initiated by private general practitioners. Mean PPI treatment duration was 24.6 days. Only a small proportion of patients in this indication had received a procedure investigating the digestive tract (2.3%) or a prescription of another anti-acid drug (2.4%), or had been hospitalized with a diagnosis of gastrointestinal disease (1.1%). Although in most cases (90.7%), PPI and NSAID therapies were initiated on the same day, the large majority of patients (79.7%) had no measurable risk factor supporting a systematic prophylactic prescription of PPI with NSAID (i.e., age > 65 years and concomitant antiplatelet, anticoagulant, or corticosteroid therapy).
In new PPI users with a co-prescription of antiplatelet/anticoagulant therapy (53.8% men; mean age 69.4 years), mean PPI treatment duration was 133.3 days, with 28.3% of patients with continuation of PPI therapy > 6 months. A procedure investigating the digestive tract had been performed in 13.7% of patients, and 7.2% had been hospitalized with a diagnosis of gastrointestinal disease.
In new PPI users with a co-prescription of corticosteroid therapy (56.8% women; mean age 52.2 years), mean PPI treatment duration was 36.5 days. Only 4.8% of patients had received a procedure investigating the digestive tract, and 2.7% had been hospitalized with a diagnosis of gastrointestinal disease.
In new PPI users with a co-prescription of chemotherapy or radiotherapy in patients with cancer (58.8% women; mean age 63.9 years), mean PPI treatment duration was 72.1 days. A procedure investigating the digestive tract had been performed in 18.3% of patients (mainly upper GI endoscopy), and 10.3% had been hospitalized with a diagnosis of gastrointestinal disease (mainly gastritis or duodenitis).
New PPI users treated for other documented digestive lesions (52.9% women; mean age 53.8 years) had a diagnosis of gastritis or duodenitis in 60.3% of cases, GERD (20.3%), esophagitis (13.2%), or other (6.2%). Prescriptions were mostly initiated by private general practitioners or hepato-gastroenterologists. Mean treatment duration was 89.4 days. At the time of PPI initiation, a large majority (91.4%) received a procedure investigating the digestive tract (mainly upper GI endoscopy) and 20.2% had a prescription of another anti-acid drug.
In new PPI users in an indeterminate indication (61.2% women; mean age 48.9 years), PPI therapy was most often initiated by a private general practitioner. Mean treatment duration was 49.8 days. A procedure investigating the digestive tract had been performed in 7.3% of patients overall (mainly upper GI endoscopy), and 8.4% among patients aged > 65 years.
Discussion
We found that more than 15 million health insurees, i.e., almost 30% of the French adult population, were PPI users in France in 2015. Nearly half of them were new users. Among new users, PPI were mainly used in co-prescription with NSAID (54%), but mostly in patients without measurable risk factors for NSAID-related complications which would support the use of a gastroprotective therapy. Based on available information, indication was undocumented for almost one-third of new PPI users.
The frequency of PPI use seems to be higher in France than in other countries, where it generally ranges between 7 and 18% [25,26,27,28,29,30]. Moreover, PPI prescriptions do not always seem to comply with the guidelines. In France, PPI misuse measured in specific subpopulations, regarding age or healthcare setting, has been reported to reach 40% to more than 80% depending on the definition used [11,12,13,14,15,16,17,18,19,20,21,22]. In our study, more than half of adult new users initiated a NSAID therapy together with a PPI, 80% of them being young patients without any measurable risk factor of GI complications. However, history of GI lesions managed in primary care setting is not always identifiable in the French National Health Data System. Thus, inappropriate use with NSAID therapy may have been overestimated. Nevertheless, misuse of gastroprotection for NSAID therapy was also observed in other countries, both regarding underuse in at-risk patients and overuse in non-at-risk patients, with figures respectively as high as 30% and 58% among NSAID users [8, 31].
For almost 2.4 million of adult new PPI users, no concomitant medication or hospital discharge diagnosis supporting the use of PPI could be identified. We can assume that at least a part of them was probably treated for uncomplicated GERD. This figure is consistent with the results of a survey estimating that nearly 3.5 million French adult subjects suffered from frequent GERD in 2003, of which less than half were prescribed a PPI [32]. Of note, although performing upper GI endoscopy is recommended before initiating GERD therapy in elderly subjects [4], only 8% of patients aged over 65 years with an indeterminate indication underwent such a procedure in our study.
Prolonged PPI therapy lasting more than 6 months was observed in 4% of new users overall, and 10% of those aged over 65 years old. However, these results probably underestimate the extent of prolonged exposure to PPIs, and higher figures were reported previously in other countries [26, 27, 30]. Divergences may be explained by the characteristics of the population studied, follow-up durations, and variations in the definitions of long-term use. In this study, the analyses were restricted to new PPI users, and did not take into account prevalent users, the latter potentially having a higher probability of being long-term users. Moreover, the definition of long-term use was constraining, as the grace period (added to the estimated end of a prescription to allow gaps between subsequent prescriptions) was relatively short (30 days).
Recent studies suggested that prolonged PPI exposure was potentially associated with serious adverse effects, including chronic kidney diseases, cardiovascular diseases, or gastrointestinal malignancies, and with a small excess of cause-specific mortality [33, 34]. PPI therapy should be avoided if not necessary, and deprescribing should be considered whenever possible through regular reevaluation. Inappropriate PPI use is a matter of great concern, especially in the elderly. This population is particularly concerned by the increase in PPI treatment duration, with a mean of 65 days per year versus 41 days overall. The combination with polypharmacy due to existing comorbidities may increase the risk of long-term PPI-related adverse outcomes [9].
This study has several strengths. First and foremost, it includes a nationwide, unselected population. Moreover, it is based on comprehensive and high-quality medical utilization claim databases. Thanks to the multiple information provided by the SNDS, a detailed description of PPI users and treatments could be obtained.
Nevertheless, results should be interpreted in light of the following limitations. First, outpatient diagnoses are not available in the SNDS. However, the large amount of individual data, regarding medications, inpatient diagnoses, medical, and laboratory procedures, allows inferring several comorbidities. This method is commonly applied in pharmacoepidemiological studies [35, 36]. Similarly, the indication for treatment is not recorded in the databases, and available information has been compiled to define the clinical context of PPI prescription, used as a proxy of the indication. Consequently, rates of potentially inappropriate prescribing were estimated rather than accurately measured. Second, posology and treatment duration were not directly available but were estimated based on dosage and number of tablets dispensed. Third, there is no guarantee on patient’s adherence to the prescription, or even that the patient actually took the drug. Finally, the SNDS does not provide information neither on inpatient nor on over-the-counter PPI use. However, in 2015, in France, 92% of PPI packs have been dispensed in private, non-hospital pharmacies (source: ANSM) and almost 97% have been prescribed by a physician (source: Open Health – Xpr-S0®). This allows us to state that estimates of PPI use based on SNDS databases are quite accurate.
In conclusion, the results of this study suggest widespread PPI use in France, not always in line with the guidelines. In particular, inappropriate co-prescription with NSAID and treatment of GERD in the absence of documentation by an upper GI endoscopy in elderly patients were frequent. PPI overuse is a major concern due to potential serious adverse events, especially in older patients and in case of prolonged exposure. Efforts should be made to limit PPI treatments to appropriate indications and durations.
References
Forgacs I, Loganayagam A (2008) Overprescribing proton pump inhibitors. BMJ 336:2–3. https://doi.org/10.1136/bmj.39406.449456.BE
National Health Service (NHS Digital). Prescription cost analysis - England, 2017 [PAS]. 2018.https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/prescription-cost-analysis-england-2017 (accessed 21 Jun 2018)
French National Agency for Medicines and Health Products Safety. Analyse des ventes de médicaments en France en 2013 [Analysis of drug sales in France in 2013] (in French). Saint-Denis, France: ANSM 2014. https://ansm.sante.fr/var/ansm_site/storage/original/application/3df7b99f8f4c9ee634a6a9b094624341.pdf (accessed 21 Jun 2019)
French National Agency for Medicines and Health Products Safety. Les antisécrétoires gastriques chez l’adulte. Recommandations de bonne pratique [Gastric antisecretory drugs in adults. Recommendations for good practice] (in French). Saint-Denis, France: AFSSAPS 2007
National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. Guidance and guidelines. Clinical guideline [CG184]. London: NICE 2014. https://www.nice.org.uk/guidance/cg184 (accessed 21 Jun 2019)
Lanza FL, Chan FKL, Quigley EMM, Practice Parameters Committee of the American College of Gastroenterology (2009) Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 104:728–738. https://doi.org/10.1038/ajg.2009.115
Strand DS, Kim D, Peura DA (2017) 25 years of proton pump inhibitors: a comprehensive review. Gut Liver 11:27–37. https://doi.org/10.5009/gnl15502
Scarpignato C, Gatta L, Zullo A, et al. Effective and safe proton pump inhibitor therapy in acid-related diseases - a position paper addressing benefits and potential harms of acid suppression. BMC Med 2016;14:179. doi:https://doi.org/10.1186/s12916-016-0718-z
Yadlapati R, Kahrilas PJ (2017) When is proton pump inhibitor use appropriate? BMC Med 15:1–4. https://doi.org/10.1186/s12916-017-0804-x
Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E (2017) The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med 37:19–24. https://doi.org/10.1016/j.ejim.2016.10.007
Levy-Neumand O, Carniaux F, Bonaz B, et al. Proton pump inhibitors in general medicine. Comparison of routine practices with marketing authorization indications. Gastroenterol Clin Biol 2007;31:78–83. doi:GCB-01-2007-31-1-0399-8320-101019-200520017
Marie I, Moutot A, Tharrasse A, Hellot MF, Robaday S, Hervé F, Lévesque H (2007) [Validity of proton pump inhibitors’ prescriptions in a department of internal medicine] (in French). Rev Med Interne 28:86–93. https://doi.org/10.1016/j.revmed.2006.09.030
Seite F, Delelis-Fanien A-S, Valero S, Pradère C, Poupet JY, Ingrand P, Paccalin M (2009) Compliance with guidelines for proton pump inhibitor prescriptions in a department of geriatrics. J Am Geriatr Soc 57:2169–2170. https://doi.org/10.1111/j.1532-5415.2009.02540.x
Villiet M, Giraudon L, Combescure C et al (2009) [Rational use of proton pump inhibitors: observational study of hospital prescriptions] (in French). J Pharm Clin 28:135–140. https://doi.org/10.1684/jpc.2009.0122
Breuvart E, Berthier S, Hars B, et al. Étude de la conformité des prescriptions d’inhibiteurs de la pompe à protons aux recommandations HAS de 2009 : étude prospective sur 208 patients [Study of the compliance of proton pump inhibitor prescriptions with the HAS recommendations 2009: a prospective study on 208 patients] (in French). Rev Médecine Interne 2013;34:A40. doi:https://doi.org/10.1016/j.revmed.2013.10.051
de Souto BP, Lapeyre-Mestre M, Mathieu C et al (2013) Prevalence and associations of the use of proton-pump inhibitors in nursing homes: a cross-sectional study. J Am Med Dir Assoc 14:265–269. https://doi.org/10.1016/j.jamda.2012.10.018
Regnier-Gavier O, Cadart H, Rolland E et al (2014) Évaluation du bon usage des inhibiteurs de la pompe à protons (IPP) [Assessment of the appropriate use of proton pump inhibitors (PPIs)] (in French). Pharm Hosp Clin 49:313. https://doi.org/10.1016/j.phclin.2014.10.023
Sauvaget L, Rolland L, Dabadie S, Desblaches J, Bernard N, Vandenhende MA, Bonnet F, Pédeboscq S, Morlat P (2015) [Survey of the prescriptions of proton pump inhibitors in patients admitted in an internal medicine ward: how is the compliance to the French guidelines?] (in French). Rev Med Interne 36:651–657. https://doi.org/10.1016/j.revmed.2015.04.014
Le Barbu M, Moutel É, Housset C et al (2016) Les inhibiteurs de la pompe à protons : encore trop de banalisation et de mésusage [Proton pump inhibitors: still too commonplace and misuse] (in French). Pharm Hosp Clin 51:353. https://doi.org/10.1016/j.phclin.2016.10.031
Schonheit C, Le Petitcorps H, Pautas É (2016) Appropriate proton pump inhibitors use in elderly outpatients according to recommendations. Geriatr Psychol Neuropsychiatr Vieil 14:383–388. https://doi.org/10.1684/pnv.2016.0623
Thorel J, McCambridge C, Piau A, et al. [Proton pump inhibitors: real indication or trivialized prescription?] (in French). Therapie 2016;71:589–593. doi:https://doi.org/10.1016/j.therap.2016.05.008
Dipanda M, Pioro L, Buttard M, d’Athis P., Asgassou S., Putot S., Deïdda M., Laborde C., Putot A., Manckoundia P. [Evaluation of the prescription of proton pump inhibitors in persons aged 75years and older in a geriatric acute-care unit] (in French). Therapie 2017;72:669–675. doi:https://doi.org/10.1016/j.therap.2017.06.003
Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646–664. doi:https://doi.org/10.1136/gutjnl-2012-302084
National Institute of Statistics and Economic Studies (Insee). Demographic balance sheet 2018. https://www.insee.fr/en/statistiques/2382609?sommaire=2382613 (accessed 21 Jun 2019)
Canadian Institute for Health Information. Prescribed Drug Spending in Canada (2016) A focus on public drug programs. Ottawa, ON: CIHI 2016 https://secure.cihi.ca/free_products/Prescribed%20Drug%20Spending%20in%20Canada_2016_EN_web.pdf (
Othman F, Card TR, Crooks CJ (2016) Proton pump inhibitor prescribing patterns in the UK: a primary care database study. Pharmacoepidemiol Drug Saf 25:1079–1087. https://doi.org/10.1002/pds.4043
Pottegård A, Broe A, Hallas J et al (2016) Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Ther Adv Gastroenterol 9:671–678. https://doi.org/10.1177/1756283X16650156
NOSOSCO (2016) Editorial group. Health Statistics for the Nordic Countries 2016. Copenhagen, Nordic Medico-Statistical Committee https://www.regeringen.ax/sites/www.regeringen.ax/files/attachments/page/health_statistics_for_the_nordic_countries_2016.pdf (
Schuiling-Veninga CCM, Hitzert A, Bos H, et al. Trends in prescribing proton pump inhibitors in the Netherlands between 2001 and 2015. Abstract from the European Drug Utilisation Research Group (EuroDURG) Conference. Glasgow, United Kingdom: 2017
Hálfdánarson ÓÖ, Pottegård A, Björnsson ES, Lund SH, Ogmundsdottir MH, Steingrímsson E, Ogmundsdottir HM, Zoega H (2018) Proton-pump inhibitors among adults: a nationwide drug-utilization study. Ther Adv Gastroenterol 11:1756284818777943. https://doi.org/10.1177/1756284818777943
Hoffmann F, Glaeske G, Schmiemann G (2015) Underuse of proton-pump inhibitors in older patients newly starting NSAID treatment. Int J Clin Pract 69:791–795. https://doi.org/10.1111/ijcp.12611
Bretagne J-F, Richard-Molard B, Honnorat C, et al. [Gastroesophageal reflux in the French general population: national survey of 8000 adults] (in French). Presse Medicale Paris Fr 1983 2006;35:23–31. doi:https://doi.org/10.1016/S0755-4982(06)74515-8
Freedberg DE, Kim LS, Yang Y-X (2017) The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 152:706–715. https://doi.org/10.1053/j.gastro.2017.01.031
Xie Y, Bowe B, Yan Y et al (2019) Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 365:l1580. https://doi.org/10.1136/bmj.l1580
Weill A, Dalichampt M, Raguideau F et al (2016) Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ 353:i2002
Bouillon K, Bertrand M, Bader G, Lucot JP, Dray-Spira R, Zureik M (2018) Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA 319:375–387. https://doi.org/10.1001/jama.2017.21269
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology: ML and RDS; data extraction: ML and TLT; data analyses: ML; interpretation of the results: all authors; writing—original draft preparation: ML; writing—review and editing: all authors; supervision: RDS.
Corresponding author
Ethics declarations
Given that data are anonymous, no informed consent is required. This study was approved by the French Data Protection Supervisory Authority (Commission Nationale de l’Informatique et des Libertés). Restrictions apply to the availability of the individual-level data supporting the findings of this study. These data so are not publicly available.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lassalle, M., Le Tri, T., Bardou, M. et al. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol 76, 449–457 (2020). https://doi.org/10.1007/s00228-019-02810-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02810-1