Abstract
Purpose
The aim of the present study was to evaluate the safety, pharmacokinetic (PK) and pharmacodynamic (PD) properties of remimazolam besylate following single ascending dose (SAD) and continuous infusion in healthy Chinese volunteers.
Methods
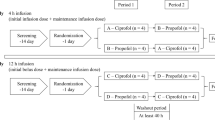
This was a randomized phase I study conducted in two parts. Part I was a double-blind, placebo- and midazolam-controlled, SAD study among healthy Chinese participants with a remimazolam dose of 0.025–0.4 mg/kg. Part II was an open-label, midazolam-controlled, continuous infusion study. Bispectral index (BIS) monitoring and Modified Observers Assessment of Alertness and Sedation (MOAA/S) score assessment were used to assess the PD properties.
Results
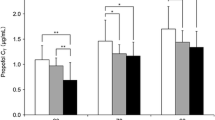
The half-life range of remimazolam was from 34.1 ± 8.1 to 59.8 ± 20.5 min in the SAD study. The sedation function was initially observed at the dose of 0.05 mg/kg remimazolam. Doses of ≥ 0.075 mg/kg exerted a peak sedation effect within 1–2 min after injection, resulting in a deeper and more rapid sedation. In the 2 h continuous infusion, remimazolam showed a deeper sedation and more rapid recovery than midazolam. For general anesthesia, an induction dosage of 0.2 mg/kg/min and a maintenance dosage of 1 mg/kg/h can achieve a satisfactory efficacy effect.
Conclusions
Remimazolam was safe and well tolerated in healthy Chinese participants. Based on the phase I clinical study, we suggest that remimazolam besylate demonstrates greater sedation and quicker recovery from sedation than midazolam.



Similar content being viewed by others
References
Cornett EM, Novitch MB, Brunk AJ, Davidson KS, Menard BL, Urman RD, Kaye AD (2018) New benzodiazepines for sedation. Best Pract Res Clin Anaesthesiol 32(2):149–164. https://doi.org/10.1016/j.bpa.2018.06.007
Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT (2011) Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol Rev 63(1):243–267. https://doi.org/10.1124/pr.110.002717
Olkkola KT, Ahonen J (2008) Midazolam and other benzodiazepines. Handb Exp Pharmacol 182:335–360. https://doi.org/10.1007/978-3-540-74806-9_16
Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ (1985) Midazolam: pharmacology and uses. Anesthesiology 62(3):310–324
Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, Wahidi M, Shojaee S, Tanner NT, Callahan SP, Feldman G, Lorch DG Jr, Ndukwu I, Pritchett MA, Silvestri GA, Investigators PAION (2019) Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest 155(1):137–146. https://doi.org/10.1016/j.chest.2018.09.015
Ulmer BJ, Hansen JJ, Overley CA, Symms MR, Chadalawada V, Liangpunsakul S, Strahl E, Mendel AM, Rex DK (2003) Propofol versus midazolam/fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol 1(6):425–432
Pambianco DJ, Cash BD (2016) New horizons for sedation: the ultrashort acting benzodiazepine remimazolam. Tech Gastrointest Endosc 18(1):22–28. https://doi.org/10.1016/j.tgie.2016.02.004
Rogers WK, McDowell TS (2010) Remimazolam, a short-acting GABA(A) receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures. IDrugs 13(12):929–937
Goudra BG, Singh PM (2014) Remimazolam: the future of its sedative potential. Saudi J Anaesth 8(3):388–391. https://doi.org/10.4103/1658-354X.136627
Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbrook GS (2007) CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology 107(1):60–66
Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD (2016) Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy 36(9):1021–1027. https://doi.org/10.1002/phar.1806
Zhou Y, Hu P, Huang Y, Nuoer S, Song K, Wang H, Wen J, Jiang J, Chen X (2018) Population pharmacokinetic/pharmacodynamic model-guided dosing optimization of a novel sedative HR7056 in Chinese healthy subjects. Front Pharmacol 9:1316. https://doi.org/10.3389/fphar.2018.01316
Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM (2012) A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. safety, efficacy, and basic pharmacokinetics. Anesth Analg 115(2):274–283. https://doi.org/10.1213/ANE.0b013e31823f0c28
Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM (2012) A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg 115(2):284–296. https://doi.org/10.1213/ANE.0b013e318241f68a
Worthington MT, Antonik LJ, Goldwater DR, Lees JP, Wilhelm-Ogunbiyi K, Borkett KM, Mitchell MC (2013) A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg 117(5):1093–1100. https://doi.org/10.1213/ANE.0b013e3182a705ae
Borett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, Wilhelm-Ogunbiyi K (2015) A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg 120(4):771–780. https://doi.org/10.1213/ANE.0000000000000548
Pambianco DJ, Borkett KM, Riff DS, Winkle PJ, Schwartz HI, Melson TI, Wilhelm-Ogunbiyi K (2016) A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc 83(5):984–992. https://doi.org/10.1016/j.gie.2015.08.062
Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, Barish CF, Pruitt R, Cash BD, Quirk D, Tiongco F, Sullivan S, Bernstein D (2018) A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc 88(3):427–437. https://doi.org/10.1016/j.gie.2018.04.2351
Bower AL, Ripepi A, Dilger J, Boparai N, Brody FJ, Ponsky JL (2000) Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc 52(2):192–196
Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL (1990) Validity and reliability of the observer’s assessment of alertness/sedation scale: study with intravenous midazolam. J Clin Psychopharmacol 10(4):244–251
Egan TD (2009) Is anesthesiology going soft? Trends in fragile pharmacology. Anesthesiology 111(2):229–230. https://doi.org/10.1097/ALN.0b013e3181ae8460
Ericsson H, Bredberg U, Eriksson U, Jolin-Mellgård A, Nordlander M, Regårdh CG (2000) Pharmacokinetics and arteriovenous differences in clevidipine concentration following a short- and a long-term intravenous infusion in healthy volunteers. Anesthesiology 92(4):993–1001. https://doi.org/10.1097/00000542-200004000-00016
Sun L, Lau CE (2001) Arteriovenous serum cocaine concentration difference after intravenous bolus injection and constant-rate infusions: relation to pharmacodynamic estimates in rats. Eur J Pharm Sci 14(4):261–269
Tuk B, Herben VM, Mandema JW, Danhof M (1998) Relevance of arteriovenous concentration differences in pharmacokinetic-pharmacodynamic modeling of midazolam. J Pharmacol Exp Ther 284(1):202–207
Olofsen E, Mooren R, van Dorp E, Aarts L, Smith T, den Hartigh J, Dahan A, Sarton E (2010) Arterial and venous pharmacokinetics of morphine-6-glucuronide and impact of sample site on pharmacodynamic parameter estimates. Anesth Analg 111(3):626–632. https://doi.org/10.1213/ANE.0b013e3181e5e8af
Acknowledgments
This study was sponsored by Yichang Humanwell Pharmaceutical Co., Ltd. We thank the contributions of WuXi AppTec Co., Ltd. for performing the concentration assays. We also thank the Department of Health Statistics, Second Military Medical University, for performing the statistical analysis.
Author information
Authors and Affiliations
Contributions
Li-e Li, Xia Ye, Xue-yuan Yang, Xia Zhao, and Yi-min Cui designed the study. Xiao-yan Sheng, Yan Liang, Xue-yuan Yang, Xia Zhao, and Yi-min Cui conducted the clinical trial. Xue-yuan Yang is the anesthesiologist of the study, who assessed the pharmacodynamic parameters and safety of the subjects. Xiao-yan Sheng and Yan Liang analyzed the results and drafted the manuscript. Li-e Li, Xia Ye, Xia Zhao, and Yi-min Cui participated in the interpretation of results and revised the manuscript. All authors read and approved the final manuscript. The authors confirm that the PI for this paper is Yi-min Cui.
Corresponding author
Ethics declarations
Conflict of interest
Li-e Li and Xia Ye are employees of Yichang Humanwell Pharmaceutical Co., Ltd., which sponsored the clinical trial. The other authors have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheng, Xy., Liang, Y., Yang, Xy. et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol 76, 383–391 (2020). https://doi.org/10.1007/s00228-019-02800-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02800-3