Abstract
Purpose
Palliative care patients often need sedation to alleviate intractable anxiety, stress, and pain. Dexmedetomidine is used for sedation of intensive care patients, but there is no prior information on its subcutaneous (SC) administration, a route that would be favored in palliative care. We compared the pharmacokinetics and cardiovascular, sympatholytic, and sedative effects of SC and intravenously (IV) administered dexmedetomidine in healthy volunteers.
Methods
An open two-period, cross-over design with balanced randomization was used. Ten male subjects were randomized to receive 1 μg/kg dexmedetomidine both IV and SC. Concentrations of dexmedetomidine and catecholamines in plasma were measured. Pharmacokinetic variables were calculated with non-compartmental methods. In addition, cardiovascular and sedative drug effects were monitored.
Results
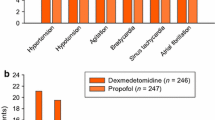
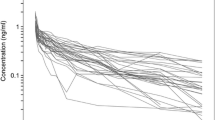
Eight subjects completed both treatment periods. Peak concentrations of dexmedetomidine were observed 15 min after SC administration (median; range 15–240). The mean bioavailability of SC dexmedetomidine was 81% (AUC0-∞ ratio × 100%, range 49–97%). The mean (SD) peak concentration of dexmedetomidine in plasma was 0.3 (0.1) ng/ml, and plasma concentrations associated with sedative effects (i.e., > 0.2 ng/ml) were maintained for 4 h after SC dosing. Plasma noradrenaline concentrations were significantly lower (P < 0.001) within 3 h after IV than after SC administration. Subjective scores for vigilance and performance were significantly lower 0–60 min after IV than SC dosing (P < 0.001 for both). The onset of the cardiovascular, sympatholytic, and sedative effects of dexmedetomidine was clearly less abrupt after SC than IV administration.
Conclusions
Dexmedetomidine is relatively rapidly and efficiently absorbed after SC administration. Subcutaneous dexmedetomidine may be a feasible alternative in palliative sedation, and causes attenuated cardiovascular effects compared to IV administration.
ClinicalTrials.gov identifier
NCT02724098. EUDRA CT number 2015-004698-34.




Similar content being viewed by others
References
Devlin JW, Roberts RJ (2011) Pharmacology of commonly used analgesics and sedatives in the ICU: benzodiazepines, propofol, and opioids. Anesthesiol Clin 29(4):567–585
Barr J, Zomorodi K, Bertaccini EJ, Shafer SL, Geller E (2001) A double-blind, randomized comparison of i.V. Lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology 95(2):286–298
Pandharipande P, Shintani A, Peterson J (2006) Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 104(1):21–26
Wujtewicz M, Maciejewski D, Misiołek H, Fijałkowska A, Gaszyński T, Knapik P, Lango R (2013) Use of dexmedetomidine in the adult intensive care unit. Anaesthesiol Intensive Ther 45(4):235–240
Aantaa R, Tonner P, Conti G, Longrois D, Mantz J, Mulier JP (2015) Sedation options for the morbidly obese intensive care unit patient: a concise survey and an agenda for development. Multidiscip Respir Med 10(1):8
Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD (2000) The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 93(2):382–394
Lodenius Å, Ebberyd A, Hårdemark Cedborg A, Hagel E, Mkrtchian S, Christensson E, Ullman J, Scheinin M, Eriksson LI, Jonsson Fagerlund M (2016) Sedation with dexmedetomidine or propofol impairs hypoxic control of breathing in healthy male volunteers: a nonblinded, randomized crossover study. Anesthesiology 125:700–715
Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, Lerman J (2006) A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology 105(6):1098–1110
Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M (1992) Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. Br J Anaesth 68(2):126–131
Talke P, Li J, Jain U, Leung J, Drasner K, Hollenberg M, Mangano DT (1995) Effects of perioperative dexmedetomidine infusion in patients undergoing vascular surgery. The study of perioperative ischemia research group. Anesthesiology 82(3):620–633
Patel CR, Engineer SR, Shah BJ, Madhu S (2012) Effect of intravenous infusion of dexmedetomidine on perioperative haemodynamic changes and postoperative recovery: a study with entropy analysis. Indian J Anaesth 56(6):542–546
Dexdor SPC (2014). URL http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002268/WC500115631.pdf
Gupta S, Singh D, Sood D, Kathuria S (2015) Role of dexmedetomidine in early extubation of the intensive care unit patients. J Anaesthesiol Clin Pharmacol 31(1):92–98
Pichot C, Ghignone M, Quintin L (2012) Dexmedetomidine and clonidine: from second- to first-line sedative agents in the critical care setting? J Intensive Care Med 27(4):219–237
Gadalla F, Spencer J (1996) Prolonged delirium after propofol. Can J Anaesth 43:87
Iirola T, Vilo S, Manner T, Aantaa R, Lahtinen M, Scheinin M, Olkkola KT (2011) Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol 67(8):825–831
Scheinin H, Karhuvaara S, Olkkola KT, Kallio A, Anttila M, Vuorilehto L, Scheinin M (1992) Pharmacodynamics and pharmacokinetics of intramuscular dexmedetomidine. Clin Pharmacol Ther 52(5):537–546
Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H (2003) Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol 56(6):691–693
Cimen ZS, Hanci A, Sivrikaya GU, Kilinc LT, Erol MK (2013) Comparison of buccal and nasal dexmedetomidine premedication for pediatric patients. Paediatr Anaesth 23(2):134–138
Declaration of Helsinki (2013) URL https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
RStudio Team (2016). RStudio: integrated development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/
Gertler R, Brown HC, Mitchell DH, Silvius EN (2001) Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 14(1):13–21
Bloor BC, Ward DS, Belleville JP, Maze M (1992) Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology 77:1134–1142
Kent CD, Kaufman BS, Lowy J (2005) Dexmedetomidine facilitates the withdrawal of ventilatory support in palliative care. Anesthesiology 103(2):439–441
Soares LG, Naylor C, Martins MA, Peixoto G (2002) Dexmedetomidine: a new option for intractable distress in the dying. J Pain Symptom Manag 24(1):6–8
Snapir A, Posti J, Kentala E, Koskenvuo J, Sundell J, Tuunanen H, Hakala K, Scheinin H, Knuuti J, Scheinin M (2006) Effects of low and high plasma concentrations of dexmedetomidine on myocardial perfusion and cardiac function in healthy male subjects. Anesthesiology 105(5):902–910
Kallio A, Scheinin M, Koulu M, Ponkilainen R, Ruskoaho H, Viinamäki O, Scheinin H (1989) Effects of dexmedetomidine, a selective alpha 2-adrenoceptor agonist, on hemodynamic control mechanisms. Clin Pharmacol Ther 46(1):33–42
Hilliard N, Brown S, Mitchinson S (2015) A case report of dexmedetomidine used to treat intractable pain and delirium in a tertiary palliative care unit. Palliat Med 29(3):278–281
O'Hara C, Tamburro RF, Ceneviva GD (2015) Dexmedetomidine for sedation during withdrawal of support. Palliat Care 9:15–18
Acknowledgements
We thank Mrs. Elina Kahra (medical laboratory technologist, Clinical Pharmacology, TYKSLAB, Hospital District of Southwest Finland, Turku, Finland) for skillful technical assistance. This study was supported financially by Turku University Hospital research fund (EVO grants 13693 and 13821), Turku, Finland.
Author information
Authors and Affiliations
Contributions
Panu Uusalo took care of the clinical phase of the study and data collection, participated in data analysis and statistical analysis and wrote the manuscript. Darin Al-Ramahi performed the analytical assays. Ida Tilli took care of the clinical phase of the study and data collection and participated in data analysis. Riku Aantaa designed the study, wrote the protocol, supervised and coordinated the clinical implementation of the study, and participated in data analysis. Mika Scheinin supervised the analytical assays, participated in data analysis and statistical analysis and writing of the manuscript. Teijo Saari designed the study, analyzed the data, performed data and statistical analysis, and wrote the manuscript. All authors materially participated in the research and/or manuscript preparation. All authors have contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
The study protocol (EudraCT 2015-004698-34, ClinicalTrials.gov identifier NCT02724098) conformed to the revised Declaration of Helsinki [20] and was approved by the Ethics Committee of the Hospital District of Southwest Finland and by the Finnish National Agency for Medicines. Written informed consent was obtained from all study participants.
Conflict of interests
M. Scheinin has been engaged in contract research for Orion Pharma, the manufacturer of dexmedetomidine. T. Saari has received honoraria for speaking at symposia organized by Orion Pharma. P. Uusalo has received a speaker’s fee from Orion Pharma. D. Al-Ramahi is currently employed by Orion Pharma, but had no affiliation with the company at the time of this study. The other authors declare no conflicts of interest.
Additional information
R. Aantaa is demised
Electronic supplementary material
ESM 1
(DOCX 15.8 kb)
Rights and permissions
About this article
Cite this article
Uusalo, P., Al-Ramahi, D., Tilli, I. et al. Subcutaneously administered dexmedetomidine is efficiently absorbed and is associated with attenuated cardiovascular effects in healthy volunteers. Eur J Clin Pharmacol 74, 1047–1054 (2018). https://doi.org/10.1007/s00228-018-2461-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2461-1