Abstract
Purpose
The purpose of this study is to describe the effectiveness of biosimilar filgrastim and original granulocyte colony-stimulating factors (G-CSFs), lenograstim and pegfilgrastim, in febrile neutropenia (FN) prevention in breast cancer patients receiving docetaxel/doxorubicin/cyclophosphamide (TAC) as adjuvant/neoadjuvant treatment and to analyze their treatment patterns.
Methods
A pharmacoepidemiology cohort study was developed in a university hospital (with 23 healthcare centers) with retrospective data collection (2012–2014). Effectiveness of G-CSFs was assessed by the FN incidence. Other parameters analyzed were as follows: moderate and severe neutropenia incidence, neutropenia-related hospitalizations, dosage, and duration. Data was analyzed using each cycle as a unit of analysis.
Results
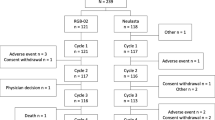
We identified 98 patients representing 518 chemotherapy cycles, 215 with original G-CSFs (35 lenograstim and 180 pegfilgrastim) and 303 with biosimilar filgrastim. The FN incidence was similar in both groups (3.7% original vs. 3.3% biosimilar; p = 0.79). No statistically significant differences were found in moderate and severe neutropenia incidence (4.7 vs. 6.3%; p = 0.43) or neutropenia-related hospitalizations (3.3 vs. 3.6%; p = 0.19). When the three drugs were evaluated separately, a higher FN incidence was observed with lenograstim than with pegfilgratim or biosimilar (p = 0.024). The dosage and duration of biosimilar were lower than lenograstim (4.9 vs. 5.7 μg/kg/day; 5 vs. 7 days; p < 0.001).
Conclusion
An abbreviated 5-day course of biosimilar filgrastim provided optimal primary prophylaxis against FN post-chemotherapy TAC in patients with breast cancer. The clinical relevance of the highest FN incidence in the lenograstim cohort needs further attention.

Similar content being viewed by others
References
Hosseinzadeh A, Thompson PR, Segal BH, Urban CF (2016) Nicotine induces neutrophil extracellular traps. J Leukoc Biol 100:1105–1112
Kourlaba G, Diopoulos MA, Pectasides D et al (2015) Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer 23:2045–2051
Kuderer NM, Dale DC, Crawford J et al (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Caggiano V, Weiss RV, Rickert TS et al (2005) Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103:1916–1924
Raposo CG, Marin AP, Barón MG (2006) Colony-stimulating factors: clinical evidence for treatment and prophylaxis of chemotherapy-induced neutropenia. Clin Transl Oncol 8:729–734
Kuderer NM, Dale DC, Crawford J et al (2007) Impact of primary prophylaxis with granulocyte colony- stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158–3167
Sung L, Nathan PC, Alibhai SMH (2007) Meta-analysis: effect of prophylactic hematopoietic colony stimulating factors on mortality and outcomes of infection. Ann Intern Med 147:400–411
Lyman GH, Kuderer NM, Djulbegovic B (2002) Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am J Med 112:406–411
Muñoz J, Gascón P, de Castro J (2012) SEOM clinical guidelines for myeloid growth factors. Clin Transl Oncol 14:491–498
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199–3212
The National Comprehensive Cancer Network (NCCN) (2016) Clinical practice guidelines in oncology: myeloid growth factors, version 2.2016. https://www.nccn.org/professionals/physician_gls/pdf/Myeloid_growth.pdf. Accessed Jan 2017
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Early Breast Cancer Trialists’ Collaborative Group. (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet 365:1687–1717
Ginés J, Sabater E, Martorell C et al (2011) Efficacy of taxanes as adjuvant treatment of breast cancer: a review and meta-analysis of randomised clinical trials. Clin Transl Oncol 13:485–498
Martin M, Pienkowski T, Mackey J et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313
Martin M, Lluch A, Segui MA et al (2006) Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol 17:1205–1212
Zamboni WC (2003) Pharmacokinetics of pegfilgrastim. Pharmacotherapy 23:9S–14S
Sveikata A, Gumbrevičius G, Seštakauskas K (2014) Comparison of the pharmacokinetic and pahrmacodynamic properties of two recombinant granulocytecolony-stimulating factor formulations after single subcutaneous administration to healthy volunteers. Medicina 50:144–149
Cortés J, Curigliano G, Diéras V (2014) Expert perspectives on biosimilar monoclonal antibodies in breast cancer. Breast Cancer Res Treat 144:233–239
Messori A, Trippoli S, Marinai C (2017) Network meta-analysis as a tool for improving the effectiveness assessment of biosimilars based on both direct and indirect evidence: application to infliximab in rheumatoid arthritis. Eur J Clin Pharmacol 73:513–514
Common Terminology Criteria for Adverse Events NCI-CTCAEv4.03 (2010) Department of health and human services, National Institutes of Health. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed Feb 2017
Holmes FA, O’Shaughnessy JA, Vukelja S et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with highrisk stage II or stage III/IV breast cancer. J Clin Oncol 20:727–731
Pfeil AM, Allcott K, Pettengell R et al (2015) Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy induced neutropenia in patients with cancer: a systematic review. Support Care Cancer 23:525–545
Cortés de Miguel S, Calleja-Hernández M, Menjón-Beltrán S et al (2015) Granulocyte colony-stimulating factors as prophylaxis against febrile neutropenia. Support Care Cancer 23:547–559
Brito M, Esteves S, Andre R et al (2016) Comparison of efficacy of primary prophylaxis with pegfilgrastim filgrastim and a biosimilar filgrastim in TAC regimen (docetaxel doxorubicin and cyclophosphamide). Support Care Cancer 24:597–603
Engert A, Griskevicius L, Zyuzgin Y et al (2009) XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia on patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymphoma 50:374–379
Del Giglio A, Eniu A, Ganea-Motan D et al (2008) XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer 8:332
Gascón P, Fuhr U, Sorgel F et al (2010) Development of a new G-CSF product based on biosimilarity assessment. Ann Oncol 21:1419–1429
Green MD, Koelbl H, Baselga J et al (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35
Mitchell S, Li X, Woods M et al (2016) Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: a systematic review. J Oncol Pharm Pract 22:702–716
Cooper KL, Madan J, Whyte S et al (2011) Granulocyte colony stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 11:404
VonMinckwitz G, Kümmel S, du Bois A et al (2008) Pegfilgrastim ± ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol 19:292–298
Weycker D, Malin J, Kim J et al (2009) Risk of hospitalization for neutropenic complications of chemotherapy in patients with primary solid tumors receiving pegfilgrastim or filgrastim prophylaxis: a retrospective cohort study. Clin Ther 31:1069–1081
Lyman GH, Abella E, Pettengell R (2014) Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol 90:190–199
Acknowledgments
We thank the Pharmacy and Oncology Department for their sustained commitment to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study received the ethical approval of the institutional review board (IRB) on June 2015 to guarantee the protection of the health information of human participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Authorship statement
All listed authors have contributed to the revision and approval of the final version of this manuscript.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Puértolas, I., Frutos Pérez-Surio, A., Alcácera, M.A. et al. Effectiveness of biosimilar filgrastim vs. original granulocyte colony-stimulating factors in febrile neutropenia prevention in breast cancer patients. Eur J Clin Pharmacol 74, 315–321 (2018). https://doi.org/10.1007/s00228-017-2365-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2365-5