Abstract
Purpose
Biosimilars are supported by limited clinical data at the time of approval. Recently, Nivestim™, a biosimilar of reference of filgrastim, was approved for prevention of chemotherapy-related febrile neutropenia (FN). To add clinical experience to this new biosimilar, we performed a study to compare the effectiveness of Nivestim™ with reference filgrastim and pegfilgrastim in FN prevention in patients receiving high-risk FN chemotherapy.
Methods
This is a comparative cohort study, with retrospective data collection. Three cohorts were identified according to the type of primary prophylaxis employed over different time periods: reference filgrastim (2004–2006), pegfilgrastim (2007–2008) and biosimilar filgrastim (2011–2012). The study included female patients with early breast cancer that received FN primary prophylaxis during (neo)adjuvant docetaxel/doxorubicin/cyclophosphamide (TAC).
Results
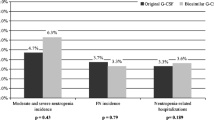
Reference filgrastim cohort included 147 patients and pegfilgrastim and biosimilar filgrastim cohorts 139 and 134 patients, respectively. FN rates per patient/cycle were 16 % (95 % confidence interval (CI) 10.2–22.5 %)/3 % (95 % CI 2.1–4.7 %) in the reference filgrastim group, 9 % (95 % CI 4.5–14.6 %)/2 % (95 % CI 1.3–3.6 %) in the pegfilgrastim group and 16 % (95 % CI 10.0–22.9 %)/4 % (95 % CI 2.5–5.3 %) in the biosimilar filgrastim cohort. The median absolute neutrophil count (ANC) at FN presentation was lower in the biosimilar group in comparison with reference filgrastim. FN episodes with ANC < 100 cells/μL were more frequent in the biosimilar group (50 %) when compared with reference filgrastim (4 %) and pegfilgrastim (6 %). No differences concerning FN complications were seen, with the exception of more chemotherapy delays in the biosimilar group when compared with pegfilgrastim.
Conclusion
No differences in biosimilar effectiveness were detected. The clinical relevance of the profound neutropenia found in the biosimilar cohort needs further attention.
Similar content being viewed by others
References
Kuderer NM, Dale DC, Crawford J et al (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Courtney DM, Aldeen AZ, Gorman SM et al (2007) Cancer associated neutropenic fever: clinical outcome and economic costs of emergency department care. Oncologist 12:1019–1026
Elting LS, Lu C, Escalante CP, Giordano SH et al (2008) Outcomes and cost of outpatient or inpatient management of 712 patients with febrile neutropenia. J Clin Oncol 26:606–611
Bonadonna G, Valagussa P (1981) Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med 304:10–15
Chirivella I, Bermejo B, Insa A et al (2009) Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat 114:479–484
Martin M, Pienkowski T, Mackey J et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313
Martín M, Seguí MA, Antón A et al (2010) Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med 363:2200–2210
Von Minckwitz G, Kümmel S, du Bois A et al (2008) Pegfilgrastim ± ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol 19:292–298
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Crawford J, Caserta C, Roila F (2010) Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol 21:248–251
Crawford J, Armitage J, Balducci L et al (2013) Myeloid growth factors. National Comprehensive Cancer Network. J Natl Compr Cancer Netw 11:1266–1290
Rajan SS, Carpenter WR, Stearns SC, Lyman GH (2013) Short-term costs associated with primary prophylactic G-CSF use during chemotherapy. Am J Manag Care 19:150–159
Waller CF, Semiglazov VF, Tjulandin S et al (2010) A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie 33:504–511
Passos-Coelho JL, Esteves S, Viera PA et al (2011) Adjuvant chemotherapy with TAC (docetaxel, doxorubicin, and cyclophosphamide) in patients with breast cancer–incidence of neutropenic fever outside clinical trials. Breast J 17:539–541
Hryniuk WM (1988) The importance of dose intensity in the outcome of chemotherapy. In: DeVita VT, Hellman S, Rosenburg SA (eds) Important advances in oncology. JB Lippincott, Philadelphia, pp 121–141
Product Monograph Neupogen: https://www.amgen.ca/Neupogen_PM.pdf
Holmes FA, Jones SE, O’Shaughnessy J et al (2002) Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol 13:903–909
Green MD, Koelbl H, Baselga J et al (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35
EMA Nivestim™ assessment report: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001142/WC500093664.pdf
Freifeld AG, Bow EJ, Sepkowitz KA et al (2011) Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52(4):427–431
González-Barca E, Fernández-Sevilla A, Carratalá J et al (1999) Prognostic factors influencing mortality in cancer patients with neutropenia and bacteremia. Eur J Clin Microbiol Infect Dis 18:539–544
Hosmer W, Malin J, Wong M (2011) Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support Care Cancer 19:333–341
Dranitsaris G, Rayson D, Vincent M et al (2008) Identifying patients at high risk for neutropenic complications during chemotherapy for metastatic breast cancer with doxorubicin or pegylated liposomal doxorubicin: the development of a prediction model. Am J Clin Oncol 31:369–374
Flowers CR, Seidenfeld J, Bow EJ et al (2013) Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 31:794–810
Timmer-Bonte JN, de Boo TM, Smit HJ et al (2005) Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch randomized phase III study. J Clin Oncol 23:7974–7984
Kamioner D, Fruehauf S, Maloisel F et al (2013) Study design: two long-term observational studies of the biosimilar filgrastim Nivestim™ (Hospira filgrastim) in the treatment and prevention of chemotherapy-induced neutropenia. BMC Cancer 13:547
Acknowledgments
The study was performed while the third author was employed at the original institution (first affiliation), but this author has recently moved to a new employer (third affiliation).
Funding
None.
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brito, M., Esteves, S., André, R. et al. Comparison of effectiveness of biosimilar filgrastim (Nivestim™), reference Amgen filgrastim and pegfilgrastim in febrile neutropenia primary prevention in breast cancer patients treated with neo(adjuvant) TAC: a non-interventional cohort study. Support Care Cancer 24, 597–603 (2016). https://doi.org/10.1007/s00520-015-2818-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2818-2