Abstract
Purpose
To investigate the impact of valproic acid (VPA) and genetic polymorphism of the major metabolizing enzyme (UGT1A4, UGT2B7) of lamotrigine (LTG) and VPA on LTG concentration in Chinese epileptic children.
Methods
Three single nucleotide polymorphisms (UGT1A4*3, UGT2B7 -161C > T and UGT2B7*2) were analyzed by polymerase chain reaction-restriction fragment length polymorphism or direct DNA sequencing. The concentrations of LTG and VPA were measured by high-performance liquid chromatography (HPLC) and fluorescence polarization immunoassay, respectively. The adjusted concentration of LTG was defined as the concentration-to-dose-ratio (CDRLTG). Data analysis was performed using IBM SPSS Statistics 21.0.
Results
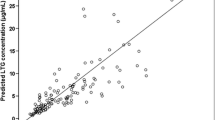
A total of 56 patients treated with LTG as monotherapy and 158 patients treated with LTG plus VPA were included in this study. In the polytherapy group, LTG concentration showed a good linear relationship with gender, age, daily LTG dose, VPA concentration, and UGT1A4*3 polymorphism, but had no relationship with the polymorphism of UGT2B7 -161C > T or UGT2B7*2. Moreover, LTG concentration and CDRLTG for the UGT1A4*3 were higher compared to UGT1A4*1 (LTG: 7.24 ± 3.51 vs 5.26 ± 3.27 μg/mL, p = 0.001; CDRLTG: 2.75 ± 1.02 vs 2.14 ± 0.96 μg/mL per mg/kg, p < 0.001, respectively). In the monotherapy group, there was no statistical difference between UGT1A4*3 and UGT1A4*1 in LTG concentration or CDRLTG. The patients in the polytherapy group were divided into two subgroups according to VPA concentration (lower/higher: 10–50/50–125 μg/mL). CDRLTG values of the patients carrying the UGT1A4*3 genotype were higher compared to UGT1A4*1*1 (2.86 ± 1.03 vs 2.22 ± 0.94 μg/mL per mg/kg, p = 0.001) only when the VPA concentration was higher.
Conclusions
UGT1A4*3 polymorphism had an effect on LTG concentration only with VPA co-administration, and the effect was remarkable when VPA concentration was higher.

Similar content being viewed by others
References
Moeller JJ, Rabey SR, Sadler RM (2009) Lamotrigine-valproic acid combination therapy for medically refractory epilepsy. Epilepsia 50:475–479
Kanner AM, Frey M (2000) Adding valproate to lamotrigine: a study of their pharmacokinetic interaction. Neurology 55:588–591
Weintraub D, Buchsbaum R, Resor SR et al (2005) Effect of antiepileptic drug comedication on lamotrigine clearance. Arch Neurol 62:1432–1436
Gidal BE, Anderson GD, Rutecki PR et al (2000) Lack of an effect of valproate concentration on lamotrigine pharmacokinetics in developmentally disabled patients with epilepsy. Epilepsy Res 42:23–31
Rowland A, Elliot DJ, Williams JA et al (2006) In vitro characterization of lamotrigine N2-glucuronidation and the lamotrigine-valproic acid interaction. Drug Metab Dispos 34:1055–1062
Chung JY, Cho JY, Yu KS et al (2008) Pharmacokinetic and pharmacodynamic interaction of lorazepam and valproic acid in relation to UGT2B7 genetic polymorphism in healthy subjects. Clin Pharmacol Ther 83:595–600
Biton V (2006) Pharmacokinetics, toxicology and safety of lamotrigine in epilepsy. Expert Opin Drug Metab Toxicol 2:1009–1018
Tukey RH, Strassburg CP (2001) Genetic multiplicity of the human UDP-glucuronosyltransferases and regulation in the gastrointestinal tract. Mol Pharmacol 59:405–414
Mackenzie PI, Bock KW, Burchell B et al (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15:677–685
Magdalou J, Herber R, Bidault R et al (1992) In vitro N-glucuronidation of a novel antiepileptic drug, lamotrigine, by human liver microsomes. J Pharmacol Exp Ther 260:1166–1173
Argikar UA, Remmel RP (2009) Variation in glucuronidation of lamotrigine in human liver microsomes. Xenobiotica 39:355–363
Ketter TA, Frye MA, Cora-Locatelli G et al (1999) Metabolism and excretion of mood stabilizers and new anticonvulsants. Cell Mol Neurobiol 19:511–532
Jin C, Miners JO, Lillywhite KJ et al (1993) Complementary deoxyribonucleic acid cloning and expression of a human liver uridine diphosphate-glucuronosyltransferase glucuronidating carboxylic acid containing drugs. J Pharmcol Exp Ther 264:475–479
Argikar UA, Remmel RP (2009) Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab Dispos 37:229–236
Gulcebi MI, Ozkaynakcı A, Goren MZ et al (2011) The relationship between UGT1A4 polymorphism and serum concentration of lamotrigine in patients with epilepsy. Epilepsy Res 95:1–8
Zhou J, Argikar UA, Remmel RP (2011) Functional analysis of UGT1A4 (P24T) and UGT1A4 (L48V) variant enzymes. Pharmacogenomics 12:1671–1679
Blanca Sánchez M, Herranz JL, Leno C et al (2010) UGT2B7_-161C>T polymorphism is associated with lamotrigine concentration-to-dose ratio in a multivariate study. Ther Drug Monit 32:177–184
Takekuma Y, Takenaka T, Yamazaki K et al (2007) Stereoselective metabolism of racemic carvedilol by UGT1A1 and UGT2B7, and effects of mutation of these enzymes on glucuronidation activity. Biol Pharm Bull 30:2146–2153
Takekuma Y, Takenaka T, Kiyokawa M (2006) Contribution of polymorphisms in UDP-glucuronosyltransferase and CYP2D6 to the individual variation in disposition of carvedilol. J Pharm Pharm Sci 9:101–112
Haina W, Lingmin Y, Su Z (2011) Characterizing the effect of UDP-glucuronosyltransferase (UGT) 2B7 and UGT1A9 genetic polymorphisms on enantioselective glucuronidation of flurbiprofen. Biochem Pharmacol 82:1757–1763
Sawyer MB, Innocenti F, Das S et al (2003) A pharmacogenetic study of uridine diphosphate-glucuronosyltransferase 2B7 in patients receiving morphine. Clin Pharmacol Ther 73:566–574
Gidal BE, Sheth R, Parnell J et al (2003) Evaluation of VPA dose and concentration effects on lamotrigine pharmacokinetics: implications for conversion to lamotrigine monotherapy. Epilepsy Res 57:85–93
Hakooz N, Alzubiedi S, Yousef AM et al (2012) UDP-glucuronosyltransferase 1A4 (UGT1A4) polymorphisms in a Jordanian population. Mol Biol Rep 39:7763–7768
Saito K, Moriya H, Sawaguchi T et al (2006) Haplotype analysis of UDP-glucuronocyltransferase 2B7 gene (UGT2B7) polymorphisms in healthy Japanese subjects. Clin Biochem 39:303–308
Lin GF, Guo WC, Chen JG et al (2005) An association of UDP-glucuronosyltransferase 2B7 C802T (His268Tyr) polymorphism with bladder cancer in benzidine-exposed workers in China. Toxicol Sci 85:502–506
Guillemette C (2003) Pharmacogenomics of human UDP-glucuronosyltransferase enzymes. Pharmacogenomics J 3:136–158
Thibaudeau J, Le´pine J, Tojcic J et al (2006) Characterization of common UGT1A8, UGT1A9, and UGT2B7 variants with different capacities to inactivate mutagenic 4-hydroxylated metabolites of estradiol and estrone. Cancer Res 66:125–133
Innocenti F, Liua W, Fackenthal D et al (2008) Single nucleotide polymorphism discovery and functional assessment of variation in the UDP-glucuronosyltransferase 2B7 gene. Pharmacogenet Genomics 18:683–697
Ross JR, Rutter D, Welsh K et al (2005) Clinical response to morphine in cancer patients and genetic variation in candidate genes. Pharmacogenomics J 5:324–336
Peterkin VC, Bauman JN, Goosen TC et al (2007) Limited influence of UGT1A1*28 and no effect of UGT2B7*2 polymorphisms on UGT1A1 or UGT2B7 activities and protein expression in human liver microsomes. Br J Clin Pharmacol 64:458–468
Mao M, Skogh E, Scordo MG et al (2012) Interindividual variation in olanzapine concentration influenced by UGT1A4 L48V polymorphism in serum and upstream FMO polymorphisms in cerebrospinal fluid. J Clin Psychopharmacol 32:287–289
Mori A, Maruo Y, Iwai M et al (2005) UDP-glucuronosyltransferase 1A4 polymorphisms in a Japanese population and kinetics of clozapine glucuronidation. Drug Metab Dispos 33:672–675
Miyagi SJ, Collier AC (2007) Pediatric development of glucuronidation: the ontogeny of hepatic UGT1A4. Drug Metab Dispos 35:1587–1592
Strassburg CP, Strassburg A, Kneip S et al (2002) Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 50:259–265
Acknowledgments
This project was supported by a grant from the National Natural Science Foundation of China (No. 81302857) and Liaoning Province Natural Science Foundation of China (No.2013021079). We are appreciative of all the participants in this study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Zhao, L., Wang, Q. et al. Influence of valproic acid concentration and polymorphism of UGT1A4*3, UGT2B7 -161C > T and UGT2B7*2 on serum concentration of lamotrigine in Chinese epileptic children. Eur J Clin Pharmacol 71, 1341–1347 (2015). https://doi.org/10.1007/s00228-015-1925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1925-9