Abstract
Purpose
The aim of the study was to develop a European list of potentially inappropriate medications (PIM) for older people, which can be used for the analysis and comparison of prescribing patterns across European countries and for clinical practice.
Methods
A preliminary PIM list was developed, based on the German PRISCUS list of potentially inappropriate medications and other PIM lists from the USA, Canada and France. Thirty experts on geriatric prescribing from Estonia, Finland, France, the Netherlands, Spain and Sweden participated; eight experts performed a structured expansion of the list, suggesting further medications; twenty-seven experts participated in a two-round Delphi survey assessing the appropriateness of drugs and suggesting dose adjustments and therapeutic alternatives. Finally, twelve experts completed a brief final survey to decide upon issues requiring further consensus.
Results
Experts reached a consensus that 282 chemical substances or drug classes from 34 therapeutic groups are PIM for older people; some PIM are restricted to a certain dose or duration of use. The PIM list contains suggestions for dose adjustments and therapeutic alternatives.
Conclusions
The European Union (EU)(7)-PIM list is a screening tool, developed with participation of experts from seven European countries, that allows identification and comparison of PIM prescribing patterns for older people across European countries. It can also be used as a guide in clinical practice, although it does not substitute the decision-making process of individualised prescribing for older people. Further research is needed to investigate the feasibility and applicability and, finally, the clinical benefits of the newly developed list.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Appropriate prescribing for older people is a public health concern, and several assessment tools are available for its evaluation. Most of the tools focus on pharmacological appropriateness of prescribing [1]; they address various aspects of appropriateness, including overprescribing of medications that are clinically not indicated, omission of medications that are needed, and incorrect prescriptions of medications that may be indicated [2]. The term “potentially inappropriate medications (PIM) for older people” has been used to refer to those drugs which should not be prescribed for this population because the risk of adverse events outweighs the clinical benefit, particularly when there is evidence in favour of a safer or more effective alternative therapy for the same condition [3, 4].
The prevalence of inappropriate prescribing and/or use of PIM has been analysed by several authors and ranges from 20 to 79 % depending on the population studied, the setting or country, and the specific tool used [5–10]. Inappropriate prescribing and use of PIM can be associated with adverse outcomes such as adverse drug events [11–13], hospitalisation [6, 14] and death [15].
A recently published systematic review identified 46 tools or criteria for assessing inappropriate prescribing [16]. A prior systematic review identified 14 criteria specific for individuals aged 65 and older [1]. Generally, the assessment tools have been developed based on expert opinion due to the lack of high-quality studies on the use of drugs in older people [17], although some tools have additionally used a literature search [18, 19]. Criteria have been classified into explicit or implicit or mixed approach [1]. Explicit criteria are generally lists of medications or criteria which can be applied with little or no clinical judgement but do not address individual differences between patients [2]. Implicit criteria are based on the judgement of a professional and are person-specific [20], requiring individual patient data for application, however, they are time-consuming and more dependent on the user [2]. No single ideal tool has been identified so far, but each tool seems to have its strengths and weaknesses, and the choice of a tool may depend on the purpose of use (i.e. daily practice, research) and availability of data [16].
Assessment tools are being used increasingly for the evaluation of prescribing quality in older people, but their application cannot substitute the individual assessment of prescribing appropriateness [16]. One of the limitations of the tools is the fact that the majority was developed following country-specific guidelines, national drug markets and prescribing habits, hence, limiting their transferability to other countries [1, 21]. For instance, the German PRISCUS list of potentially inappropriate medications, a purely explicit list, defines 83 PIM drugs, of which twelve are not on the drug market in France, the USA and Canada. However, there are 124 drugs on the PIM lists of these countries which are not part of the German PRISCUS list, because seventy of them are not on the German drug market and many others are almost never used [22]. To the best of our knowledge, no assessment tool covers the drug markets of several European countries and could thus enable the analysis of European databases.
The present study was conceived when planning to analyse the prescription of PIM among a European cohort of older people with dementia participating in the RightTimePlaceCare study [23]. The primary aim of our study was to develop an expert-consensus list of potentially inappropriate medications covering the drug markets of seven European countries, which can be used for the analysis of potentially inappropriate prescription patterns in and across several European countries. Additionally, the list should be applicable in clinical practice to alert health care professionals to the likelihood of inappropriate prescribing, possible dose adjustments required and therapeutic alternatives.
Methods
A research team consisting of a clinical pharmacologist, a pharmacist, a nursing scientist and a geriatrician planned and coordinated the development of the European Union (EU)(7)-PIM list. Two members of the research team were developers of the German PRISCUS list [22]. The study comprised five consecutive phases:
-
1.
Preparation of a preliminary PIM list. We prepared a preliminary PIM list which contained 85 PIM (82 active substances plus one combination of active substances and two different preparations of one substance) from the German PRISCUS list [22] and 99 PIM from the French [3], American [24, 25] and Canadian [26] lists. These tools have been used in research to evaluate the prescription of PIM and factors associated with PIM use [5, 6, 14, 27–29]. The main reason for each drug being PIM was formulated using the information provided by the original lists. This process was supported by a comprehensive literature search. The anatomical therapeutic chemical (ATC) code classification system was used (2011) [30].
-
2.
Recruitment of experts on geriatric prescribing/pharmacotherapy. We established a collaboration with the Seventh Framework European project RightTimePlaceCare [23], a project aiming to develop best practice recommendations for dementia care throughout Europe. The consortium partners of this project supported the recruitment of experts on geriatric prescribing or pharmacotherapy in their respective countries. Thirty-three experts from six European countries agreed to participate; they came from Finland (n = 3), Estonia (n = 9), the Netherlands (n = 4), France (n = 2), Spain (n = 7) and Sweden (n = 8). The following professions were represented as follows: geriatricians (n = 14), pharmacists (n = 3), clinical pharmacologists (n = 7) and other medical specialists (n = 9). Experts were sent information documents describing the aims, concepts and steps of the study and were asked whether they preferred to participate in the expansion phase (phase 3), in the Delphi survey (phase 4), or in both.
-
3.
Expansion of the preliminary PIM list. We asked thirteen experts representing the six countries to expand the preliminary PIM list by adding drugs that they considered should be PIM and which were not represented, paying special attention to those drugs available on their respective countries’ markets. Expansion of the preliminary list was Internet-based and concluded in May 2012.
-
4.
Two-round Delphi survey. A two-round Delphi survey was performed [31]. The first Delphi round took place between October and December 2012, and the second Delphi round between March and May 2013. In the first round, we asked 29 experts to assess each drug of the preliminary expanded list for appropriateness by using a 1–5 points Likert scale where “1” represented “I strongly agree that the drug is potentially inappropriate for older people”; “2”, “I agree that the drug is potentially inappropriate for older people”; “3”, “average/neutral/undecided”; “4”, “I disagree that the drug is potentially inappropriate for older people”; “5”, “I strongly disagree that the drug is potentially inappropriate for older people”; and “0”, “no answer; I do not feel qualified to answer”. Experts were asked to provide suggestions for dose adjustments and safer therapeutic alternatives for those drugs judged as inappropriate. Experts were free to insert additional comments and were invited to expand the list with any further drugs they considered to be PIM.
In the second Delphi round, we asked 28 experts to assess the appropriateness of those drugs classified as questionable PIM during the first round (see “Expert agreement and statistics”), as well as the further suggestions for PIM made by the experts during the first Delphi round, and also eight drugs appearing in the recently published updated Beers list [18]. Some PIM concepts were adapted taking the experts’ suggestions made during the first Delphi round into account. The additional suggestions for PIM were given a justification as to why they may be classified as PIM, taking published data into consideration when necessary. Again, experts assessed the appropriateness of these drugs and were asked to provide dose adjustments, therapeutic alternatives, and to insert additional comments if necessary. Drugs were classified into PIM, non-PIM and questionable PIM (see “Expert agreement and statistics”).
-
5.
Preparation of the final PIM list. Dose adjustments and drug alternatives suggested by the experts during the Delphi survey were compiled and included in the EU(7)-PIM list, prioritising in each case those made by the higher number of experts. Suggestions were complemented, if necessary, with information available from the other PIM lists and from Micromedex® [32], a commercially available database which contains comprehensive information on drug use. We identified those drugs for which some discussion issues raised by the experts still remained open and those drugs where inconsistency in the results was identified after checking the literature. In order to solve these problems, a reduced number of experts (n = 12) was invited to participate in the last brief survey which took place in September 2013.
Expert agreement and statistics
Several approaches have been suggested in the literature to define expert agreement within Delphi surveys [31]. In this study, after the first and second Delphi rounds, we calculated the means, the corresponding 95 % confidence intervals (CI) and the medians of all Likert scores given to each drug; expert agreement was considered if the CI of the mean score for each drug did not cross over the value 3. Thus, each drug was classified into PIM (if both the mean value of the score and the upper limit of the CI were lower than 3), non-PIM (if both the mean value of the score and the lower limit of the CI exceeded 3) and questionable PIM (if the CI was on both sides of the value 3). Statistical calculations were performed with SPSS, version 21.0.
Results
The preliminary PIM list contained 184 drugs (including two combinations of two drugs) and preparations (e.g. sustained-release preparations of oxybutynine). Eight of the 13 invited experts (62 %) participated in the expansion phase and suggested 75 additional drugs and preparations. Twenty-six out of the 29 invited experts (90 %) participated in the first Delphi round, and 24 out of the 28 invited experts (86 %) participated in the second Delphi round. Two experts from Spain and three experts from Finland chose to collaborate together in two teams to provide their assessments in both Delphi rounds. All the 12 experts invited participated in the last brief survey.
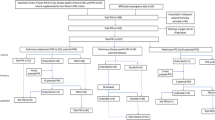
Figure 1 shows the development process of the list. In the first Delphi round, experts assessed 259 drugs and preparations, of which the majority (n = 234) were classified as PIM and only one drug as non-PIM. In the second Delphi round, experts assessed 79 drugs and preparations, comprising 23 questionable PIM, 47 further suggestions by experts, eight additional drugs from the updated Beers list [18] and one drug (naproxen) judged as PIM for which the main reason for PIM was adapted taking recent published data and experts’ comments into consideration. Again, 31 drugs and preparations remained as questionable PIM and 46 drugs were classified as PIM. Overall, after the third brief survey, 282 drugs and preparations were classified as PIM, 29 as questionable PIM and three as non-PIM.
The level of agreement between experts varied in the assessment of appropriateness. For example, experts reached consensus for diazepam being PIM with a mean Likert score of 1.61, confidence interval between 1.32 and 1.89, and median of 2. Consensus was reached also for digoxin being PIM (mean Likert score 2.19; confidence interval 1.57–2.81; median 2), but in this case, the Likert scores ranged from 1 to 5. No consensus was reached on the appropriateness of some drugs such as metamizole, which was classified as questionable PIM. For this drug, the disparity seemed to be in part due to the experts’ country of origin, since the majority of the Spanish experts considered metamizole to be appropriate when used in adequate doses, whereas the majority of Finnish experts considered this drug to be clearly inappropriate.
The last brief survey consisted of 11 questions with multiple-choice answers and covered issues regarding 13 drugs. The questions covered mostly dose-related issues commented by the experts during the survey which remained open (four drugs) and inconsistencies in the results identified after checking the literature (three drugs). Additionally, the research group asked the experts to provide their opinion on the use of three drugs. Finally, the research group did minimal corrections in the PIM which needed experts’ approval (three drugs). All of the issues could be solved.
Table 1 displays an abbreviated version of the EU(7)-PIM list, with the 72 PIM most frequently identified among the participants of the RightTimePlaceCare survey [23], a European cohort of older people with dementia (data not shown).
Appendix 1 shows the complete EU(7)-PIM list, which comprises 275 chemical substances (i.e. 7-digit ATC codes; e.g. amitriptyline) including two combinations of two chemical substances, plus seven drug classes (i.e. 5-digit ATC codes; e.g. triptans), belonging to 55 therapeutic classes (i.e. 4-digit ATC codes; e.g. antidepressants) and 34 therapeutic groups (i.e. 3-digit ATC codes; e.g. the nervous system). Some PIM concepts are dose-related (e.g. zopiclone used at doses higher than 3.75 mg/day) or defined by length of use (e.g. proton-pump inhibitors used longer than 8 weeks) or drug regimen (e.g. insulin, sliding scale). Appendix 1 contains also information on the number of experts who assessed each PIM, the mean, median and standard deviation of the scores given by experts to each drug (Likert scale), and the results of the compilation and selection of suggestions for dose adjustments and therapeutic alternatives. Furthermore, Appendix 1 shows two categories of those drugs (active substances characterised by their ATC code) on the EU-PIM list that are included also on other PIM lists. Category A means that precisely this active substance is named as a PIM which should be avoided in older people. Category B means that (i) this active substance is characterised as a PIM only in the case of certain clinical conditions or co-morbidities or (ii) this active substance is not specifically named but considered as a PIM drug class (e.g. anticholinergics or long-acting benzodiazepines). This information refers to six international PIM lists or criteria [3, 18, 19, 22, 26, 33] and shows that 24 drugs do not appear as PIM in any of the other lists, while the rest varies from appearing in one list only to appearing in all the lists.
The full lists of questionable PIM and non-PIM and the results of their assessments are presented in Appendix 2 and 3, respectively.
Discussion
We developed the EU(7)-PIM list in order to analyse the prescription patterns of potentially inappropriate medication (PIM) across several European countries, and more specifically among the people with dementia participating in the RightTimePlaceCare Seventh Framework European project [23]. We also aimed to develop a list that would be applicable in clinical practice. The development of the EU(7)-PIM list took several international PIM lists (i.e. the German PRISCUS list [22], the American Beers list [18, 24, 25], the Canadian list [26], and the French list [3]) into consideration, as well as further drugs suggested by experts on geriatric prescribing from seven European countries who belonged to different professions.
The EU(7)-PIM list can be seen as a screening tool for the identification of PIM for older people across many European countries. We have covered several regions of Europe including Finland and Sweden in Scandinavia, France and Spain in southern Europe, Germany and the Netherlands in central Europe, and Estonia in eastern Europe. As shown by Fialová et al. [5], the prevalence of PIM use in several European countries varies widely, depending on the PIM criteria set. Thus, the creation of a PIM list suitable for pharmacoepidemiological studies and clinical use in Europe seems to be mandatory. Attempts are being undertaken to develop prescribing quality indicators which are useful for the electronic monitoring of the quality of prescribing in older people in Europe [34], and the EU(7)-PIM list could represent a part of this.
We expect the EU(7)-PIM list to be a sensitive tool because of its inclusive development process. In contrast, other tools have been seen to be less sensitive, motivating some authors to use two or three assessment tools for the assessment of PIM use in their populations in order to increase the sensitivity [5, 6, 35, 36].
We aimed at developing a list which can be used even if the clinical information available is minimal. Therefore, we chose to develop explicit PIM criteria, restricted to drugs or drug classes, in some instances restricted to high doses or prolonged treatment duration. Thus, the EU(7)-PIM list is suitable for pharmacoepidemiological applications using administrative databases or surveys without any clinical information about the individuals concerned.
To the best of our knowledge, this is the first list focusing on chemical substances and requiring only a small amount of clinical data for its application that has been developed taking into account several existing PIM lists and European markets, and that has been consented by experts from different European countries. This is also one of the few lists including suggestions for dose adjustments and therapeutic alternatives. Furthermore, the list enables a distinction between different drugs belonging to the same pharmacological subgroup and provides different suggestions for each of them. The recently published screening tool of older person’s prescriptions (STOPP)/screening tool to alert doctors to right treatment (START) criteria for potentially inappropriate prescribing for older people (version 2) were developed also with the participation of a European panel of experts [19]. However, these criteria often consider as PIM the use of pharmacological subgroups (e.g. thiazide diuretics) within specific clinical contexts (e.g. history of gout, or current significant hypokalaemia). Thus, the application of the START/STOPP criteria (both versions 1 and 2) [4, 19] requires clinical information, making these criteria more suitable in the clinical context for a comprehensive drug review of individual patients.
The development process of the EU(7)-PIM list resembles those of most other PIM lists, such as the French list [3], the German PRISCUS list [22], the Austrian PIM list [37], but also the most recent Beers list [18]. One major aspect of criticism of all PIM lists is that the classification of PIM is usually done without using evidence derived from randomised, controlled trials and relies on the expertise of the participants in the Delphi process [38]. However, this is partially justified by the lack of evidence on drug efficacy and safety in older people, due to their low enrolment in clinical trials [17]. In our study, we identified relevant literature and used it during the development process, but we did not systematically review and report it, which may be seen as a limitation.
The Delphi technique has also been criticised because of the lack of one standardised method, the difficulties in analysing the data, the difficulties in defining what an expert is, the often heterogeneous expert group, and the vague concept of consensus [38]. In order to minimise the limitations of the Delphi technique, in the present study, the characteristics of the survey were predefined (e.g. steps, consensus concept), and researchers provided experts with all necessary information to favour their engagement and participation. Researchers compiled discussion issues raised by the experts and took them into consideration for the consecutive steps of the development process.
Only seven European countries participated in the development of the EU(7)-PIM list (Estonia, Finland, France, Germany, the Netherlands, Spain and Sweden). Furthermore, the number of experts participating from some countries was limited. Certain drugs may not have been assessed for appropriateness because they were neither included in the preliminary list nor were they suggested by the experts. Certain drugs were classified as PIM with a lower level of expert agreement than others; some disagreements seemed related to the experts’ country of origin, which may show that there are international differences in prescription patterns or attitudes. Regular updates of the list should take into consideration the inclusion of other European markets, the changes in the drug markets, the prescribing tendencies, and above all, the new existing evidence.
The application of the EU(7)-PIM list cannot substitute the individual assessment of prescribing appropriateness, which should take into account other aspects such as the aims of the treatment, individual responses, and the older person’s functional level, values and preferences, among others [39]. This limitation has been recognised in the literature with regard to most tools assessing appropriateness of prescription [16]. Despite its limitations, the concept of PIM suggests that their use should be associated with less favourable outcomes. Indeed, the use of PIM has been found associated with a higher rate of adverse drug reactions in several studies, as reported in a systematic review [40], with some variations depending on the settings studied. Other authors have suggested an association between PIM use and other adverse outcomes such as injuries [41] and hospitalisation [6, 14]. A limited number of studies on interventions involving the use of some of these tools have suggested benefits in terms or relevant outcomes [42–44]. However, according to a recent systematic review, it is unclear whether such interventions result in clinically significant improvements, although benefits in terms of reducing inappropriate prescribing may exist [45].
Future research should study whether the use of PIM according to the EU(7)-PIM list shows any association with clinically relevant outcomes for older people, and whether the application of the list is associated with any benefits, both in a population and on individual levels. The acceptability of the list among health professionals should also be investigated, including the usefulness of the suggestions for drug adjustments and therapeutic alternatives.
In conclusion, the EU(7)-PIM list is an expert-consensus list of potentially inappropriate medications for older people, which was developed taking into consideration the medications appearing in six country-specific PIM lists, as well as medications used in seven European countries. It is an explicit list of chemical substances and contains suggestions for dose adjustments and therapeutic alternatives. It can be applied as a screening tool to identify potentially inappropriate medications in databases where little clinical information is available and in individual data. It can also be used for international comparisons of the prescription patterns of PIMs and may be used as a guide in the clinical practice. The application of the EU(7)-PIM list is a first step towards the identification of areas of improvement in both individual and population levels and towards the harmonisation of the prescription quality throughout Europe.
References
Dimitrow MS, Airaksinen MS, Kivela SL, Lyles A, Leikola SN (2011) Comparison of prescribing criteria to evaluate the appropriateness of drug treatment in individuals aged 65 and older: a systematic review. J Am Geriatr Soc 59(8):1521–1530
Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, Hanlon JT (2007) Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet 370(9582):173–184
Laroche ML, Charmes JP, Merle L (2007) Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol 63(8):725–731
Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D (2008) STOPP (screening tool of older person’s prescriptions) and START (screening tool to alert doctors to right treatment). Consensus validation. Int J Clin Pharmacol Ther 46(2):72–83
Fialova D, Topinkova E, Gambassi G, Finne-Soveri H, Jonsson PV, Carpenter I, Schroll M, Onder G, Sorbye LW, Wagner C, Reissigova J, Bernabei R (2005) Potentially inappropriate medication use among elderly home care patients in Europe. JAMA 293(11):1348–1358
Reich O, Rosemann T, Rapold R, Blozik E, Senn O (2014) Potentially inappropriate medication use in older patients in Swiss managed care plans: prevalence, determinants and association with hospitalization. PLoS One 9(8), e105425. doi:10.1371/journal.pone.0105425
Gallagher P, O’Mahony D (2008) STOPP (screening tool of older persons’ potentially inappropriate prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing 37(6):673–679
Gallagher P, Lang PO, Cherubini A, Topinkova E, Cruz-Jentoft A, Montero Errasquin B, Madlova P, Gasperini B, Baeyens H, Baeyens JP, Michel JP, O’Mahony D (2011) Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol 67(11):1175–1188
Garcia-Gollarte F, Baleriola-Julvez J, Ferrero-Lopez I, Cruz-Jentoft AJ (2012) Inappropriate drug prescription at nursing home admission. J Am Med Dir Assoc 13(1):83 e9–83 e15
Mann E, Haastert B, Frühwald T, Sauermann R, Hinteregger M, Hölzl D, Keuerleber S, Scheuringer M, Meyer G (2014) Potentially inappropriate medication in older persons in Austria: a nationwide prevalence study. Eur Geriatr Med 5(6):399–405
Chang CM, Liu PY, Yang YH, Yang YC, Wu CF, Lu FH (2005) Use of the Beers criteria to predict adverse drug reactions among first-visit elderly outpatients. Pharmacotherapy 25(6):831–838
Passarelli MC, Jacob-Filho W, Figueras A (2005) Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging 22(9):767–777
Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D (2011) Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 171(11):1013–1019
Price SD, Holman CD, Sanfilippo FM, Emery JD (2014) Association between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patients. Ann Pharmacother 48(1):6–16
Gosch M, Wortz M, Nicholas JA, Doshi HK, Kammerlander C, Lechleitner M (2014) Inappropriate prescribing as a predictor for long-term mortality after hip fracture. Gerontology 60(2):114–122
Kaufmann CP, Tremp R, Hersberger KE, Lampert ML (2014) Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol 70(1):1–11
Campbell SM, Cantrill JA (2001) Consensus methods in prescribing research. J Clin Pharm Ther 26(1):5–14
American Geriatrics Society Beers Criteria Update Expert P (2012) American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 60(4):616–631
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P (2014) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. doi:10.1093/ageing/afu145
Shelton PS, Fritsch MA, Scott MA (2000) Assessing medication appropriateness in the elderly: a review of available measures. Drugs Aging 16(6):437–450
Marshall MN, Shekelle PG, McGlynn EA, Campbell S, Brook RH, Roland MO (2003) Can health care quality indicators be transferred between countries? Qual Saf Health Care 12(1):8–12
Holt S, Schmiedl S, Thurmann PA (2010) Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int 107(31-32):543–551
Verbeek H, Meyer G, Leino-Kilpi H, Zabalegui A, Hallberg IR, Saks K, Soto ME, Challis D, Sauerland D, Hamers JP (2012) A European study investigating patterns of transition from home care towards institutional dementia care: the protocol of a RightTimePlaceCare study. BMC Public Health 12:68
Beers MH (1997) Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med 157(14):1531–1536
Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH (2003) Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 163(22):2716–2724
McLeod PJ, Huang AR, Tamblyn RM, Gayton DC (1997) Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ 156(3):385–391
Schubert I, Kupper-Nybelen J, Ihle P, Thurmann P (2013) Prescribing potentially inappropriate medication (PIM) in Germany’s elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol Drug Saf 22(7):719–727
Montastruc F, Gardette V, Cantet C, Piau A, Lapeyre-Mestre M, Vellas B, Montastruc JL, Andrieu S (2013) Potentially inappropriate medication use among patients with Alzheimer disease in the REAL.FR cohort: be aware of atropinic and benzodiazepine drugs! Eur J Clin Pharmacol 69(8):1589–1597
Fick DM, Maclean JR, Rodriguez NA, Short L, Heuvel RV, Waller JL, Rogers RL (2004) A randomized study to decrease the use of potentially inappropriate medications among community-dwelling older adults in a southeastern managed care organization. Am J Manag Care 10(11 Pt 1):761–768
The anatomical therapeutic chemical classification system and the defined daily dose. Internet database. WHO Collaborating Centre for Drug Statistics Methodology, Norwegian Institute of Public Health. Nydalen, Oslo, Norway. http://www.whocc.no/atc_ddd_index/. Accessed 17 February 2015
Jones J, Hunter D (1995) Consensus methods for medical and health services research. BMJ 311(7001):376–380
Truven health micromedex solutions. Internet database. Greenwood Village, Colorado, USA. http://www.micromedexsolutions.com/home/dispatch. Accessed 31 October 2014
Finnish Medicines Agency. Database of medication for the elderly. Internet database. http://www.fimea.fi/medicines/medicines_assesment/. Accessed 19 February 2015
European Science Foundation (2015). Workshops detail: enhancing the quality and safety of pharmacotherapy in old age. http://www.esf.org/coordinating-research/exploratory-workshops/workshops-list/workshops-detail.html?ew=13023. Accessed 20 April 2015
Siebert S, Elkeles B, Hempel G, Kruse J, Smollich M (2013) The PRISCUS list in clinical routine. Practicability and comparison to international PIM lists. Z Gerontol Geriatr 46(1):35–47
Elseviers MM, Vander Stichele RR, Van Bortel L (2014) Quality of prescribing in Belgian nursing homes: an electronic assessment of the medication chart. Int J Qual Health Care 26(1):93–99
Mann E, Bohmdorfer B, Fruhwald T, Roller-Wirnsberger RE, Dovjak P, Duckelmann-Hofer C, Fischer P, Rabady S, Iglseder B (2012) Potentially inappropriate medication in geriatric patients: the Austrian consensus panel list. Wien Klin Wochenschr 124(5-6):160–169
Marriott J, Stehlik P (2012) A critical analysis of the methods used to develop explicit clinical criteria for use in older people. Age Ageing 41(4):441–450
Steinman MA, Hanlon JT (2010) Managing medications in clinically complex elders: there’s got to be a happy medium. JAMA 304(14):1592–1601
Jano E, Aparasu RR (2007) Healthcare outcomes associated with beers’ criteria: a systematic review. Ann Pharmacother 41(3):438–447
Bauer TK, Lindenbaum K, Stroka MA, Engel S, Linder R, Verheyen F (2012) Fall risk increasing drugs and injuries of the frail elderly—evidence from administrative data. Pharmacoepidemiol Drug Saf 21(12):1321–1327
Hill-Taylor B, Sketris I, Hayden J, Byrne S, O’Sullivan D, Christie R (2013) Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther 38(5):360–372
Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y (2014) Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 62(9):1658–1665
Blozik E, Born AM, Stuck AE, Benninger U, Gillmann G, Clough-Gorr KM (2010) Reduction of inappropriate medications among older nursing-home residents: a nurse-led, pre/post-design, intervention study. Drugs Aging 27(12):1009–1017
Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, Hughes C (2014) Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 10, Cd008165. doi:10.1002/14651858.CD008165.pub3
ATC classification with defined daily dose DDD. Internet database. German Institute of Medical Documentation and Information. https://www.dimdi.de/dynamic/en/contact.html. Accessed 17 February 2015
Acknowledgments
We are very thankful to the following experts on geriatric prescribing and/or pharmacotherapy for their participation in the development process of the EU(7)-PIM list: Anti Kalda (University of Tartu, Tartu, Estonia), Jana Lass (University of Tartu, Tartu, Estonia), Mai Rosenberg (University of Tartu, Tartu, Estonia), Peeter Saadla (Tartu University Hospital, Tartu, Estonia), Kai Saks (University of Tartu, Tartu, Estonia), Gennadi Timberg (West-Tallinn Central Hospital and MedTIM Private Urological Clinic, Tallinn, Estonia), Tiina Uuetoa (East-Tallinn Central Hospital, Tallinn, Estonia), Risto Huupponen (University of Turku, Turku, Finland), Paula Viikari (Turku City Hospital, Turku, Finland), Matti Viitanen (University of Turku, Turku, Finland), François Montastruc (Toulouse University Hospital, Toulouse, France), Antoine Piau (Toulouse University Hospital, Toulouse, France), Froukje Boersma (University Medical Center Groningen, Groningen, the Netherlands), Paul A. F. Jansen (Expertise Centre Pharmacotherapy in Old Persons (EPHOR) Utrecht, the Netherlands), Rob van Marum (Jeroen Bosch Hospital, ‘s Hertogenbosch, VU University Medical Center, Amsterdam, the Netherlands), Jos M. G. A. Schols (University of Maastricht, Maastricht, the Netherlands), Eva Delgado-Silveira (University Hospital Ramón y Cajal, Madrid, Spain), Antonio Fernandez Moyano (San Juan de Dios del Aljarafe Hospital, Sevilla, Spain), Francesc Formiga (University Hospital of Bellvitge, Barcelona, Spain), Elisabet de Jaime (Parc de Salut Mar, Barcelona, Spain), Ramón Miralles (Parc de Salut Mar, Barcelona, Spain), María Muñoz (University Hospital Ramón y Cajal, Madrid, Spain), Olga Vázquez (Parc de Salut Mar, Barcelona, Spain), Robert Eggertsen (University of Gothenburg, Sahlgrenska Academy, Gothenburg, Sweden), Peter Engfeldt (Örebro University, Örebro, Sweden), Tommy Eriksson (Lund University, Lund, Sweden), Johan Fastbom (Karolinska Institute and Stockholm University, Stockholm, Sweden), Annika Kragh (Lund University, Lund, Sweden), Patrik Midlöv (Center for Primary Health Care Research, Lund University, Malmö, Sweden), and Anders Wimo (Karolinska Institute, Stockholm, Sweden).
We thank Dr Stefanie Holt-Noreiks and Dr Simone Bernard, University of Witten/Herdecke, for their scientific contribution throughout the project. We also thank Ms Vivienne Krause, University of Witten/Herdecke, who checked the manuscript and the EU(7)-PIM list for the English language; Ms Klaaßen-Mielke, Ruhr University Bochum, who contributed in the coding and plausibility checks of the list; and Ms Malin Wörster, who contributed in comparing the EU(7)-PIM list with other international lists. We are thankful to the RTPC Consortium partners who supported us to establish the contact to the experts. Finally, we thank the University of Witten/Herdecke for supporting the project and the Sociedad Española de Geriatría y Gerontología (Spanish Society of Geriatric Medicine and Gerontology) for supporting the work of the first author of this study with a grant.
Compliance with ethical standards
ᅟ
Conflict of interest
The authors declare that they have no competing interests.
Funding
This study was supported by a research grant of the University of Witten/Herdecke.
Contributions of authors’ statement
Petra A Thürmann (PAT) and Gabriele Meyer (GM) conceptualised the study and applied for funding. Anna Renom-Guiteras (ARG) and PAT prepared the work documents during the development process. ARG recruited the experts and coordinated the expansion phase, the Delphi survey and the last brief questionnaire. PAT and ARG prepared the final version of the EU(7)-PIM list. ARG drafted the manuscript, supported by GM and PAT. All the authors critically reviewed and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Renom-Guiteras, A., Meyer, G. & Thürmann, P.A. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 71, 861–875 (2015). https://doi.org/10.1007/s00228-015-1860-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1860-9