Abstract
Purpose
The aim of this study was to compare the systemic exposure of lercanidipine (Zanidip) after oral administration in the fasted state and 15 min before food intake (meals) to investigate if the recommendations in the Summary of Product Characteristics (SPC) with respect to the intake of meals are adequate.
Methods
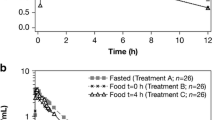
The results of three pilot bioequivalence studies performed to develop a lercanidipine generic product, where Zanidip was administered consistently as reference product in the fasted state or 15 min before a standard breakfast, were compared to estimate the drug–food interaction and the similarity of the methods of administration defined in the SPC.
Results
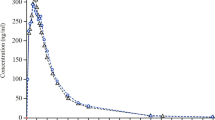
The ingestion of a standard (non-high-fat, non-high-calorie) meal 15 min after drug intake increased the area under the concentration–time curve (AUC0-t) of S-lercanidipine by 1.78-fold [90% confidence interval (CI) 1.48–2.15, P < 0.0001] and the maximum concentration (Cmax) of S-lercanidipine by 1.82-fold (90% CI 1.46–2.28, P < 0.0001). These values are close to the twofold increase that has been described when Zanidip was taken immediately after a carbohydrate-rich meal. Higher levels would be expected with a high-fat, high-calorie meal.
Conclusions
As intake with a carbohydrate-rich meal is not recommended in the SPC of Zanidip because a twofold difference was considered to be clinically relevant, the intake of lercanidipine only 15 min before food intake does not seem to be consistent with this recommendation. The Marketing Authorisation Holder should clarify the dosing instructions in relation to meals and identify a sufficient time-lapse to ensure an exposure similar to that obtained in phase III clinical efficacy studies.
Similar content being viewed by others
References
Bang LM, Chapman TM, Goa KL (2003) Lercanidipine: a review of its efficacy in the management of hypertension. Drugs 63(22):2449–2472
Borghi C (2005) Lercanidipine in hypertension. Vasc Health Risk Manag 1(3):173–182
Burnier M, Pruijm M, Wuerzner G (2009) Treatment of essential hypertension with calcium channel blockers: what is the place of lercanidipine? Exp Opin Drug Metab Toxicol 5(8):981–987
Beckey C, Lundy A, Lutfi N (2007) Lercanidipine in the treatment of hypertension. Ann Pharmacother 41(3):465–473
Jabor VA, Coelho EB, Lanchote VL (2004) Enantioselective pharmacokinetics of lercanidipine in healthy volunteers. J Chromatogr B Analyt Technol Biomed Life Sci 813(1–2):343–346
Barchielli M, Dolfini E, Farina P, Leoni B, Targa G, Vinaccia V, Tajana A (1997) Clinical pharmacokinetics of lercanidipine. J Cardiovasc Pharmacol 29[Suppl 2]:S1–S15
Medicine and Healthcare products Regulatory Agency (2006) Summary of Products Characteristics of Zanidip 10 mg tablets. Available at: http://www.medicines.org.uk/emc/medicine/17624/SPC. Accessed 1 May 2011
Medicine and Healthcare products Regulatory Agency (2010) Public Assessment Report of Zanidip Novum 8 mg and 16 mg film coated tablets. 2 February 2010. Available at: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con099740.pdf. Accessed 1 May 2011
Medsafe, New Zealand Medicines and Medical Devices Safety Authority (2002). Zanidip Data Sheet. 23 January 2002. Available at: http://www.medsafe.govt.nz/profs/datasheet/z/Zanidiptab.htm. Accessed 1 May 2011
Therapeutic Goods Administration (2010) Lercanidipine Sandoz Product Information. 30 September 2010. Available at: http://www.medicines.org.au/files/smplercs.pdf. Accessed 1 May 2011
Medicine and Healthcare products Regulatory Agency (2009) Public assessment report of lercanidipine hydrochloride 10 mg and 20 mg film coated tablets. 22 December 2009. Available at: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con120461.pdf. Accessed 1 May 2011
Higaki K, Choe SY, Lobenberg R, Welage LS, Amidon GL (2008) Mechanistic understanding of time-dependent oral absorption based on gastric motor activity in humans. Eur J Pharm Biopharm 70(1):313–325
Society of Hospital Pharmacists of Hong Kong Administration (2009) Zanidip misprints dosage instraction. Posted 24 November 2009. Available at: https://sites.google.com/a/shphk.org.hk/main/pharmacists/medsafe. Accessed 1 May 2011
Acknowledgements
We thank the Complutense University and Madrid Community Administration and its research group number 910939.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript presents the personal opinion of the author Alfredo García-Arieta and does not necessarily represent the views or policy of the Spanish Agency for Medicines and Health Care Products.
Rights and permissions
About this article
Cite this article
Álvarez, C., Gómez, E., Simón, M. et al. Differences in lercanidipine systemic exposure when administered according to labelling: in fasting state and 15 minutes before food intake. Eur J Clin Pharmacol 68, 1043–1047 (2012). https://doi.org/10.1007/s00228-012-1215-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1215-8