Abstract
Purpose
To determine the effects of food on the pharmacokinetics of sublingual asenapine.
Methods
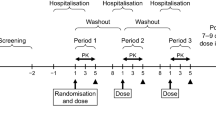
Healthy male volunteers (n = 26, age 19–53 years) randomly received a single sublingual dose of asenapine 5 mg after ≥10 h fasting (Treatment A, reference), after a high-fat meal (Treatment B) and after ≥10 h fasting with a high-fat meal at 4 h post-dose (Treatment C). Blood samples were drawn over 72 h to measure asenapine plasma concentrations. Effects of food intake on asenapine pharmacokinetics were assessed using bioequivalence criteria and evaluated using a compartmental modelling analysis.
Results
Compared with the reference, mean asenapine exposure (AUC0-last and AUC0-∞) was approximately 20 % lower after intake of a high-fat meal prior to dosing, whereas Cmax decreased by only about 10 %. When a high-fat meal was taken 4 h post-dose in the fasting state, asenapine concentrations were similar to the reference during the first 4 h post-dose. After the meal intake, asenapine concentrations decreased quickly for several hours. Compartmental modelling indicated that a transient 2.5-fold increase in asenapine clearance after eating could explain the asenapine concentration–time profiles for both food regimens.
Conclusions
To our knowledge, this is the first study investigating the effect of food upon the sublingual administration of a drug. A high-fat meal taken before or 4 h post-dose of sublingual asenapine indirectly caused a transient increase in liver blood flow that resulted in a temporal increase in asenapine clearance. As the effects on asenapine exposure were small and not clinically relevant, no additional restrictions are required for the timing of food intake in relation to asenapine dosing.




Similar content being viewed by others
References
Schering Corporation, a subsidiary of Merck & Co., Inc (2010) Saphris® (asenapine sublingual tablets). Full prescribing information. Available at: http://www.merck.com/product/usa/pi_circulars/s/saphris/saphris_pi.pdf. Accessed 30 Nov 2012
Reuters (2012) Merck & Co wins EU agency support for antipsychotic. Available at: http://www.reuters.com/article/idUSLDE65O0RE20100625. Accessed 30 Nov 2012
Shahid M, Walker GB, Zorn SH, Wong EH (2009) Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol 23:65–73
Bartlett JA, van der Voort Maarschalk K (2012) Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech 13:1110–1115
Dogterom P, Timmer C, Spaans E, deVries R, Vanvilet A, Peeters P (2012) Asenapine safety, tolerability, and pharmacokinetics after single and multiple doses in healthy volunteers. Clin Pharmacol in Drug Dev 1:131–143
van de Wetering-Krebbers SF, Jacobs PL, Kemperman GJ, Spaans E, Peeters PA, Delbressine LP, van Iersel ML (2011) Metabolism and excretion of asenapine in healthy male subjects. Drug Metab Dispos 39:580–590
de Boer T, Meulman E, Meijering H, Wieling J, Dogterom P, Lass H (2012) Quantification of asenapine and three metabolites in human plasma using liquid chromatography-tandem mass spectrometry with automated solid-phase extraction: application to a phase I clinical trial with asenapine in healthy male subjects. Biomed Chromatogr 26:156–165
Dogterom P, Hulskotte E, Gerrits M, Timmer C, Sitsen JM, DeGreef H, Spaans E, Peeters PA (2009) Asenapine pharmacokinetics: influence of cytochrome P450 modulators and UDP-glucuronyltransferase inhibition (abstract). Eur Neuropsychopharmacol 18[Suppl 4]:S452–S453
Peeters P, Bockbrader H, Spaans E, Dogterom P, Lasseter K, Marbury T, Gibson GL, de Greef R (2011) Asenapine pharmacokinetics in hepatic and renal impairment. Clin Pharmacokinet 50:471–481
Hulskotte E, Spaans E, Timmer C, Schrodter A, Machielsen C, Schnabel P, van den Heuvel M, de Greef R, Peeters P (2009) Effects of water intake and smoking on absorption of sublingually administered asenapine. Presented at: American Psychiatric Association Annual Meeting. San Francisco, California
van Griensven JM, Burggraaf KJ, Gerloff J, Gunzler WA, Beier H, Kroon R, Huisman LG, Schoemaker RC, Kluft K, Cohen AF (1995) Effects of changing liver blood flow by exercise and food on kinetics and dynamics of saruplase. Clin Pharmacol Ther 57:381–389
U.S. Department of Health and Human Services (2012) Dietary guidelines for Americans. Available at: http://www.dietaryguidelines.gov. Accessed 30 Nov 2012
Food and Drug Administration, Center for Drug Evaluation and Research (CDER) (2002) Guidance for Industry Food-Effect Bioavailability and Fed Bioequivalence Studies. Available at: http://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126833.pdf. Accessed 1 May 2013
Beal SL, Sheiner LB (1992) NONMEM user’s guides. NONMEM Project Group, University of California, San Francisco
Ette EI, Ludden TM (1995) Population pharmacokinetic modeling: the importance of informative graphics. Pharm Res 12:1845–1855
Maitre PO, Buhrer M, Thomson D, Stanski DR (1991) A three-step approach combining Bayesian regression and NONMEM population analysis: application to midazolam. J Pharmacokinet Biopharm 19:377–384
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic–pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm 20:511–528
Prohn M, de Greef R, Chapel S, Kerbusch T (2009) Population pharmacokinetics of asenapine in patients with schizophrenia or bipolar disorder. Eur Neuropsychopharmacol 19:S542–S543
Gaiani S, Bolondi L, Li BS, Santi V, Zironi G, Barbara L (1989) Effect of meal on portal hemodynamics in healthy humans and in patients with chronic liver disease. Hepatology 9:815–819
Orrego H, Mena I, Baraona E, Palma R (1965) Modifications in hepatic blood flow and portal pressure produced by different diets. Am J Dig Dis 10:239–248
Svensson CK, Edwards DJ, Mauriello PM, Barde SH, Foster AC, Lanc RA, Middleton E Jr, Lalka D (1983) Effect of food on hepatic blood flow: implications in the “food effect” phenomenon. Clin Pharmacol Ther 34:316–323
Burggraaf J, Schoemaker HC, Cohen AF (1996) Assessment of changes in liver blood flow after food intake—comparison of ICG clearance and echo-Doppler. Br J Clin Pharmacol 42:499–502
Daneshmend TK, Roberts CJ (1982) The influence of food on the oral and intravenous pharmacokinetics of a high clearance drug: a study with labetalol. Br J Clin Pharmacol 14:73–78
Elvin AT, Cole AF, Pieper JA, Rolbin SH, Lalka D (1981) Effect of food on lidocaine kinetics: mechanism of food-related alteration in high intrinsic clearance drug elimination. Clin Pharmacol Ther 30:455–460
Gupta SK, Manfro RC, Tomlanovich SJ, Gambertoglio JG, Garovoy MR, Benet LZ (1990) Effect of food on the pharmacokinetics of cyclosporine in healthy subjects following oral and intravenous administration. J Clin Pharmacol 30:643–653
Hahn RG, Norberg A, Gabrielsson J, Danielsson A, Jones AW (1994) Eating a meal increases the clearance of ethanol given by intravenous infusion. Alcohol Alcohol 29:673–677
Schoemaker RC, Burggraaf J, Cohen AF (1998) Assessment of hepatic blood flow using continuous infusion of high clearance drugs. Br J Clin Pharmacol 45:463–469
Acknowledgements
This study was conducted by C&T Paradigm, Antwerp, Belgium in 2005 under the responsibility of Luc Tritsmans, MD. Medical writing and editorial assistance was provided by Bagi Ravishankar, PhD, of PPSI, New Jersey (a PAREXEL company) and was funded by Merck & Co. Inc., Whitehouse Station, New Jersey, USA.
Funding/Conflict of Interest Statement
This study was funded by Merck & Co. Inc., Whitehouse Station, New Jersey, USA, and all authors were employees of Merck & Co at the time the study was conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dogterom, P., de Greef, R. & Peeters, P.A.M. The effect of food on the high clearance drug asenapine after sublingual administration to healthy male volunteers. Eur J Clin Pharmacol 71, 65–74 (2015). https://doi.org/10.1007/s00228-013-1587-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1587-4