Abstract
Purpose
The objective of this study was to determine whether the so-called “shift” or “drift” problem might occur when generic anti-epileptic drugs are interchanged, and thus to assess if generic anti-epileptic drugs are interchangeable and can be used in an efficacious and safe way on the basis of their bioequivalence to one and the same reference product.
Methods
The bioequivalence of topiramate and gabapentin generics was evaluated. For proper interstudy comparison, individual exposure data (AUC and Cmax) for each bioequivalence study present in the registration dossier was normalized based on the absolute exposure data of one of two innovators. The exposure-normalized plasma concentration curves of the generic product arms between studies were compared, providing indirect evidence of bioequivalence of the different generics. Additionally, comparisons were made for generic–generic as well as innovator–innovator exchange based on absolute exposure data from individual bioequivalence studies.
Results
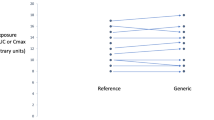
In almost all cases, estimated 90% confidence intervals of the AUC and Cmax ratios for generic–generic interchange were within the routine 80–125% criterion. When absolute, non-corrected exposure data were used for this interstudy comparison, in a number of cases 90% confidence intervals outside the 80–125% criterion were found upon interchanging generics from two studies. However, a similar pattern of 90% confidence intervals outside the 80–125% criterion was observed for the comparison of innovator arms, despite the fact that the innovator was identical in all studies.
Conclusion
Our results strongly indicate that the so-called drifting problem upon generic–generic substitution does not result in important differences in exposure upon exchanging topiramate generics or gabapentin generics.


Similar content being viewed by others
References
Krämer G, Biraben A, Carreno M, Guekht A, de Haan GJ, Jedrzejczak J, Josephs D, van Rijckevorsel K, Zaccara G (2007) Current approaches to the use of generic antiepileptic drugs. Epilepsy Behav 11:46–52. doi:10.1016/j.yebeh.2007.03.014
Versantvoort CHM, Maliepaard M, Lekkerkerker JFF (2008) Generics: what is the role of registration Authorities. Neth J Med 66:62–66
Anderson S, Hauck WW (1996) The transitivity of bioequivalence testing: potential for drift. Intl J Clin Pharm and Therap 34:369–374
Anonymous (1987) For and against generic prescribing. Drug Ther Bull 25:93–95
Besag FMC (2000) Is generic prescribing acceptable in epilepsy? Drug Saf 23:173–182
Commission on Epidemiology and Prognosis, International League Against Epilepsy (1993) Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 34:592–596
WHO. Epilepsy fact sheet No999. World Health Organization (January 2009). Available at www.who.int/mediacentre/factsheets/fs999/en/index.html. Accessed 11 November 2010
Welty TE (2007) Pharmacy and generic substitution of antiepileptic drugs: missing in action? Ann Pharmacother 41:1065–1068. doi:10.1345/aph.1K076
Berg MJ (2007) What's the problem with generic antiepileptic drugs? A call to action. Neurology 68:1245–1246. doi:10.1212/01.wnl.0000262876.37269.8b
Heany DC, Sander JW (2007) Antiepileptic drugs: generic versus branded treatments. Lancet Neurol 6:465–468. doi:10.1016/S1474-4422(07)70105-9
Burkhardt RT, Leppik IE, Blesi K, Scott S, Gapany SR, Cloyd JC (2004) Lower phenytoin serum levels in persons switched from brand to generic phenytoin. Neurology 63:1494–1496
Makus KG, McCormick J (2007) Identification of adverse reactions that can occur on substitution of generic for branded lamotrigine in patients with epilepsy. Clin Ther 29:334–341. doi:10.1016/j.clinthera.2007.02.005
Crawford P, Feely M, Guberman A, Kramer G (2006) Are there potential problems with generic substitution of antiepileptic drugs? A review of issues. Seizure 15:165–176. doi:10.1016/j.seizure.2005.12.010
Maliepaard M, Hekster YA, Kappelle A, van Puijenbroek EP, Elferink AJ, Welink J, Gispen-de Wieden CC, Lekkerkerker JFF (2009) Requirements for generic anti-epileptic medicines: a regulatory perspective. J Neurol 256:1966–1971. doi:10.1007/s00415-009-5231-2
Berg MJ, Gross RA, Tomaszewski KJ, Zingaro WM, Haskins LS (2008) Generic substitution in the treatment of epilepsy: case evidence of breakthrough seizures. Neurology 71:525–530. doi:10.1212/01.wnl.0000319958.37502.8e
Krämer GJ, Steinhoff BJ, Feucht M, Pfäfflin M, May TW (2007) Experience with generic drugs in epilepsy patients: an electronic survey of members of the German, Austrian and Swiss branches of the ILAE. Epilepsia 48:609–611. doi:10.1111/j.1528-1167.2007.01084_1.x
Wilner AN (2004) Therapeutic equivalency of generic antiepileptic drugs: results of a survey. Epilepsy Behav 5:995–998. doi:10.1016/j.yebeh.2004.05.011
Berg MJ, Gross RA (2006) Physicians and patients perceive that generic drug substitution of anti-epileptic drugs can cause breakthrough seizures—results from a U.S. survey. First North American Regional Epilepsy Congress: 60th Annual Meeting of the American Epilepsy Society; 1–5 December 2006; San Diego, California. Abstract 2.105.
Andermann F, Duh MS, Gosselin A, Paradis PE (2007) Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia 48:464–469. doi:10.1111/j.1528-1167.2007.01007.x
Zachry WM III, Doan QD, Clewell JD, Smith BJ (2008) Case-control analysis of ambulance, emergency room, or inpatient hospital events for epilepsy and antiepileptic drug formulation changes. Epilepsia 50:493–500. doi:10.1111/j.1528-1167.2008.01703.x
Kesselheim AS, Stedman MR, Bubrick EJ, Gagne JJ, Misono AS, Lee JL, Brookhart MA, Avorn J, Shrank WH (2010) Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs 70:605–621. doi:10.2165/10898530-000000000-00000
Stewart BH, Kugler AR, Thompson PR, Bockbrader HN (1993) A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res 10:276–281
Davit BM, Nwakama PE, Buehler GJ, Conner DP, Haidar SH, Patel DT, Yang Y, Yu LX, Woodcock J (2009) Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother 43:1583–1597. doi:10.1345/aph.1M141
Nightingale SL (1998) Therapeutic equivalence of generic drugs: letter to health practitioners, 1998. Available at: www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ucm073182.htm. Accessed 11 November 2010
Gidal BE (2009) Bioequivalence of antiepileptic drugs: how close is close enough? Curr Neurol Neurosci Rep 9:333–337
Acknowledgements
All authors, except Nikola Banishki, are appointed to the Dutch Medicines Evaluation Board (MEB-CBG). Nikola Banishki has been working as a student at this Agency. All authors had access to the application dossiers filed at the Regulatory Agency. The opinions of the authors expressed in this paper do not necessarily reflect those of the Dutch Medicines Evaluation Board MEB-CBG.
Conflict of interest
None of the authors has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix Fig. 1
(PDF 12 kb)
Appendix Fig. 2
(PDF 18 kb)
ESM 1
(DOC 27 kb)
Rights and permissions
About this article
Cite this article
Maliepaard, M., Banishki, N., Gispen-de Wied, C.C. et al. Interchangeability of generic anti-epileptic drugs: a quantitative analysis of topiramate and gabapentin. Eur J Clin Pharmacol 67, 1007–1016 (2011). https://doi.org/10.1007/s00228-011-1041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1041-4