Abstract
Purpose
Compared with genetic factors, drug interactions were largely unexplored in warfarin pharmacogenetic studies. This study sought to systematically investigate the effects of genetic polymorphisms of VKORC1, STX4A, CYP2C9, CYP3A4, and GGCX and interacting drugs on the initial responses to warfarin in Chinese patients with heart valve replacement (HVR).
Methods
A retrospective study was conducted in 809 patients starting warfarin therapy after HVR. The relationships between 12 polymorphisms plus 47 drugs and primary outcomes of the time to the first international normalized ratio (INR) ≥ 1.8 and the time to the first INR > 3.5 and the secondary outcomes of the proportion of time INR < 1.8, the proportion of time INR > 3.5, and the daily warfarin dose in the first 28 days after the initiation of warfarin treatment were analyzed.
Results
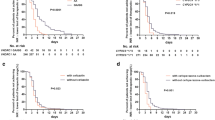
Genetic polymorphisms and interacting drugs could significantly affect the primary and secondary outcomes. The time to the first INR ≥ 1.8 was significantly influenced by the body surface area (BSA), VKORC1 g.3588G > A allele, and CYP2C9*3 allele, with hazard ratio (HR; 95% confidence interval [CI]) of 0.34 (0.17–0.66), 2.71 (2.2–3.35) and 1.43 (1.07–1.93) respectively. The time to the first INR > 3.5 was affected not only by BSA, VKORC1 g.3588G > A allele, and CYP2C9*3 allele with HR (95%CI) of 0.26 (0.07–0.99), 2.76 (1.61–4.72), and 3.09 (2.02–4.74) respectively, but also by age and interacting drugs, including fluconazole, amiodarone, and simvastatin with HR (95%CI) of 1.02 (1.01–1.04), 2.66 (1.16–6.08), 1.78 (1.17–2.73), and 5.33 (1.67–16.96) respectively.
Conclusions
Not only VKORC1 and CYP2C9 genotypes, but also interacting drugs, had a significant impact on the variability of the initial response to warfarin.


Similar content being viewed by others
References
Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, Milligan PE, Grice G, Lenzini P, Rettie AE, Aquilante CL, Grosso L, Marsh S, Langaee T, Farnett LE, Voora D, Veenstra DL, Glynn RJ, Barrett A, McLeod HL (2008) Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther 84:326–331
Black DJ, Kunze KL, Wienkers LC, Gidal BE, Seaton TL, McDonnell ND, Evans JS, Bauwens JE, Trager WF (1996) Warfarin-fluconazole. II. A metabolically based drug interaction: in vivo studies. Drug Metab Dispos 24:422–428
Ngui JS, Chen Q, Shou M, Wang RW, Stearns RA, Baillie TA, Tang W (2001) In vitro stimulation of warfarin metabolism by quinidine: increases in the formation of 4′- and 10-hydroxywarfarin. Drug Metab Dispos 29:877–886
Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, Kim RB, Roden DM, Stein CM (2008) Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med 358:999–1008
Nishiyama A, Kim-Mitsuyama S (2010) New approaches to blockade of the renin-angiotensin-aldosterone system: overview of regulation of the renin-angiotensin-aldosterone system. J Pharmacol Sci 113:289–291
Li C, Schwarz UI, Ritchie MD, Roden DM, Stein CM, Kurnik D (2009) Relative contribution of CYP2C9 and VKORC1 genotypes and early INR response to the prediction of warfarin sensitivity during initiation of therapy. Blood 113:3925–3930
Kotirum S, Chaiyakunapruk N, Jampachaisri K, Wattanasombat S, Rojnuckarin P (2007) Utilization review of concomitant use of potentially interacting drugs in Thai patients using warfarin therapy. Pharmacoepidemiol Drug Saf 16:216–222
Sconce EA, Khan TI, Daly AK, Wynne HA, Kamali F (2006) The impact of simvastatin on warfarin disposition and dose requirements. J Thromb Haemost 4:1422–1424
Yuen E, Gueorguieva I, Wise S, Soon D, Aarons L (2010) Ethnic differences in the population pharmacokinetics and pharmacodynamics of warfarin. J Pharmacokinet Pharmacodyn 37:3–24
Miners JO, Birkett DJ (1998) Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45:525–538
Stevenson PH (1937) Height-weight-surface formula for the estimation of surface area in Chinese subjects. Chin J Physiol 3:327–330
Dorn GW Jr (2010) Therapeutic potential of microRNAs in heart failure. Curr Cardiol Rep 12:209–215
Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, Wells PS (2005) Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 165:1095–1106
Yuan HY, Chiou JJ, Tseng WH, Liu CH, Liu CK, Lin YJ, Wang HH, Yao A, Chen YT, Hsu CN (2006) FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res 34:W635–W641
Wang TL, Li HL, Tjong WY, Chen QS, Wu GS, Zhu HT, Hou ZS, Xu S, Ma SJ, Wu M, Tai S (2008) Genetic factors contribute to patient-specific warfarin dose for Han Chinese. Clin Chim Acta 396:76–79
Kimura R, Miyashita K, Kokubo Y, Akaiwa Y, Otsubo R, Nagatsuka K, Otsuki T, Okayama A, Minematsu K, Naritomi H, Honda S, Tomoike H, Miyata T (2007) Genotypes of vitamin K epoxide reductase, gamma-glutamyl carboxylase, and cytochrome P450 2C9 as determinants of daily warfarin dose in Japanese patients. Thromb Res 120:181–186
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E (1993) A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 69:236–239
Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, Lu MJ, Hung CR, Wei CY, Chen CH, Wu JY, Chen YT (2005) A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet 14:1745–1751
Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, Bentley D, McGinnis R, Deloukas P (2007) Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 121:23–34
Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, Whittaker P, Ranganath V, Kumanduri V, McLaren W, Holm L, Lindh J, Rane A, Wadelius M, Deloukas P (2009) A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet 5:e1000433
Wysowski DK, Nourjah P, Swartz L (2007) Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 167:1414–1419
Neal JM, Kunze KL, Levy RH, O’Reilly RA, Trager WF (2003) Kiiv, an in vivo parameter for predicting the magnitude of a drug interaction arising from competitive enzyme inhibition. Drug Metab Dispos 31:1043–1048
Bavisotto LM, Ellis DJ, Milner PG, Combs DL, Irwin I, Canafax DM (2010) Tecarfarin, a novel vitamin K reductase antagonist, is not affected by CYP2C9 and CYP3A4 inhibition following concomitant administration of fluconazole in healthy participants. J Clin Pharmacol doi:10.1177/0091270010370588
Schelleman H, Bilker WB, Brensinger CM, Han X, Kimmel SE, Hennessy S (2008) Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther 84:581–588
Mootha VV, Schluter ML, Das A (2002) Intraocular hemorrhages due to warfarin fluconazole drug interaction in a patient with presumed Candida endophthalmitis. Arch Ophthalmol 120:94–95
Kerin NZ, Blevins RD, Goldman L, Faitel K, Rubenfire M (1988) The incidence, magnitude, and time course of the amiodarone-warfarin interaction. Arch Intern Med 148:1779–1781
Almog S, Shafran N, Halkin H, Weiss P, Farfel Z, Martinowitz U, Bank H (1985) Mechanism of warfarin potentiation by amiodarone: dose- and concentration-dependent inhibition of warfarin elimination. Eur J Clin Pharmacol 28:257–261
Herman D, Locatelli I, Grabnar I, Peternel P, Stegnar M, Lainscak M, Mrhar A, Breskvar K, Dolzan V (2006) The influence of co-treatment with carbamazepine, amiodarone and statins on warfarin metabolism and maintenance dose. Eur J Clin Pharmacol 62:291–296
Schelleman H, Bilker WB, Brensinger CM, Wan F, Yang YX, Hennessy S (2010) Fibrate/statin initiation in warfarin users and gastrointestinal bleeding risk. Am J Med 123:151–157
Acknowledgements
This work has been supported by the National Science Foundation of China (30772620, 81070103, 81072701), and the National Key Basic Research Program (NKBRP) of China (2006CB503806).
Conflict of interest
No conflicts to disclose.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shi-Long Zhong and Yuan Liu made same experimental contribution to the paper
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Primer sequences used in SNaPShot assay for genotyping. (DOC 37 kb)
Table S2
The impact of genotypes on the percentage of time INR < 1.8 or percentage of time INR > 3.5. (DOC 83 kb)
Table S3
The impact of concomitant interacting drugs on the percentage of time INR < 1.8 or percentage of time INR > 3.5. (DOC 71 kb)

Fig. S1
The impact of genotypes and interacting drugs on the time course of daily warfarin doses during the early phase of warfarin therapy. The daily warfarin doses were grouped according to (A) g.3588G>A genotypes in VKORC1, (B) c.1075A>C genotypes in CYP2C9, ©) with or without fluconazole, and (D) with or without amiodarone. (JPEG 656 kb)
Rights and permissions
About this article
Cite this article
Zhong, SL., Liu, Y., Yu, XY. et al. The influence of genetic polymorphisms and interacting drugs on initial response to warfarin in Chinese patients with heart valve replacement. Eur J Clin Pharmacol 67, 581–590 (2011). https://doi.org/10.1007/s00228-011-0995-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-0995-6