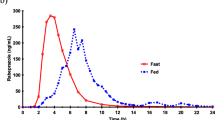
Abstract
Aims
To characterize and compare the pharmacokinetic profiles of bromazepam, omeprazole and paracetamol when administered by the oral and nasogastric routes to the same healthy cohort of volunteers.
Methods
In a prospective, monocentric, randomized crossover study, eight healthy volunteers received the three drugs by the oral (OR) and nasogastric routes (NT). Sequential plasma samples were analyzed by high-performance liquid chromatography–UV, pharmacokinetic parameters (Cmax, \({\text{AUC}}_{0 - \infty } \), t½, ke, tmax) were compared statistically, and Cmax, \({\text{AUC}}_{0 - \infty } \) and tmax were analyzed for bioequivalence.
Results
A statistically significant difference was seen in the \({\text{AUC}}_{0 - \infty } \) of bromazepam, with nasogastric administration decreasing availability by about 25%: AUCOR = 2501 ng mL−1 h; AUCNT = 1855 ng mL−1 h (p < 0.05); ratio (geometric mean) = 0.74 [90% confidence interval (CI) 0.64–0.87]. However, this does not appear to be clinically relevant given the usual dosage range and the drug’s half-life (approx. 30 h). A large interindividual variability in omeprazole parameters prevented any statistical conclusion from being drawn in terms of both modes of administration despite their similar average profile: AUCOR = 579 ng mL−1 h; AUCNT = 587 ng mL−1 h (p > 0.05); ratio (geometric mean) = 1.01 (90% CI 0.64–1.61). An extended study with a larger number of subjects may possibly provide clearer answers. The narrow 90% confidence limits of paracetamol indicate bioequivalence: AUCOR = 37 μg mL−1 h; AUCNT = 41 μg mL−1 h(p > 0.05); ratio (geometric mean) = 1.12 (90% CI 0.98–1.28).
Conclusion
The results of this study show that the nasogastric route of administration does not appear to cause marked, clinically unsuitable alterations in the bioavailability of the tested drugs.




Similar content being viewed by others
References
Randall HT (1984) Sixth annual Jonathan E. Rhoads lecture. Enteral nutrition: tube feeding in acute and chronic illness. J Parenter Enteral Nutr 8:113–136
Hébuterne X (1998) Technique de la nutrition entérale: matériel, solutions, modalités d’administration. Traité de nutrition artificielle de l’adulte. Société Française de Nutrition Clinique et Métabolisme, Paris, pp 445–463
Lochs H, Dejong C, Hammarqvist F et al (2006) ESPEN guidelines on enteral nutrition. Gastroenterol Clin Nutr 25:260–274
Berger MM, Chiolero R, Pannatier A et al (1997) A 10-year survey of nutritional support in a surgical ICU: 1986–1995. Nutrition 13:870–877
Bauer LA (1982) Interference of oral phenytoin absorption by continuous nasogastric feedings. Neurology 32:570–572
Holtz L, Milton J, Sturek JK (1987) Compatibility of medications with enteral feedings. J Parenter Enteral Nutr 11:183–186
Worden JP Jr, Wood CA Jr, Workman CH (1984) Phenytoin and nasogastric feedings. Neurology 34:132
Saklad JJ, Graves RH, Sharp WP (1986) Interaction of oral phenytoin with enteral feedings. J Parenter Enteral Nutr 10:322–323
Weinryb J, Cogen R (1989) Interaction of nasogastric phenytoin and enteral feeding solution. J Am Geriatr Soc 37:195–196
Hooks MA, Longe RL, Taylor AT et al (1986) Recovery of phenytoin from an enteral nutrient formula. Am J Hosp Pharm 43:685–688
Longe RL, Smith OB (1988) Phenytoin interaction with an oral feeding results in loss of seizure control. J Am Geriatr Soc 36:542–544
Rodman DP, Stevenson TL, Ray TR (1995) Phenytoin malabsorption after jejunostomy tube delivery. Pharmacotherapy 15:801–805
Hennessy DD (2003) Recovery of phenytoin from feeding formulas and protein mixtures. Am J Health Syst Pharm 60:1850–1852
Au Yeung SC, Ensom MH (2000) Phenytoin and enteral feedings: does evidence support an interaction? Ann Pharmacother 34:896–905
Gal P, Layson R (1986) Interference with oral theophylline absorption by continuous nasogastric feedings. Ther Drug Monit 8:421–423
Bhargava VO, Schaaf LJ, Berlinger WG et al (1989) Effect of an enteral nutrient formula on sustained-release theophylline absorption. Ther Drug Monit 11:515–519
Plezia PM, Thornley SM, Kramer TH et al (1990) The influence of enteral feedings on sustained-release theophylline absorption. Pharmacotherapy 10:356–361
Mueller BA, Brierton DG, Abel SR et al (1994) Effect of enteral feeding with ensure on oral bioavailabilities of ofloxacin and ciprofloxacin. Antimicrob Agents Chemother 38:2101–2105
Wright DH, Pietz SL, Konstantinides FN et al (2000) Decreased in vitro fluoroquinolone concentrations after admixture with an enteral feeding formulation. J Parenter Enteral Nutr 24:42–48
Kanji S, McKinnon PS, Barletta JF et al (2003) Bioavailability of gatifloxacin by gastric tube administration with and without concomitant enteral feeding in critically ill patients. Crit Care Med 31:1347–1352
Burkhardt O, Stass H, Thuss U et al (2005) Effects of enteral feeding on the oral bioavailability of moxifloxacin in healthy volunteers. Clin Pharmacokinet 44:969–976
Vincent J, Teng R, Pelletier SM et al (1998) The bioavailability of nasogastric versus tablet-form oral trovafloxacin in healthy subjects. Am J Surg 176:23S–26S
Kays MB, Overholser BR, Lagvankar S et al (2005) Effect of ensure on the oral bioavailability of gatifloxacin in healthy volunteers. Pharmacotherapy 25:1530–1535
Garcia-Encina G, Farran R, Puig S et al (1999) Validation of an automated liquid chromatographic method for omeprazole in human plasma using on-line solid-phase extraction. J Pharm Biomed Anal 21:371–382
Berger MM, Berger-Gryllaki M, Wiesel PH (2000) Intestinal absorption in patients after cardiac surgery. Crit Care Med 28:2217–2223
Commission de la Société Française des Sciences et Techniques Pharmaceutiques (2003) Validation des procédures analytiques quantitatives. Harmonisation des démarches. STP Pharma Pratiques 13:101–138
Blase E, Taylor K, Gao HY et al (2005) Pharmacokinetics of an oral drug (acetaminophen) administered at various times in relation to subcutaneous injection of exenatide (exendin-4) in healthy subjects. J Clin Pharmacol 45:570–577
Stillings M, Havlik I, Chetty M et al (2000) Comparison of the pharmacokinetic profiles of soluble aspirin and solid paracetamol tablets in fed and fasted volunteers. Curr Med Res Opin 16:115–124
Stangier J, Su CA, Fraunhofer A et al (2000) Pharmacokinetics of acetaminophen and ibuprofen when coadministered with telmisartan in healthy volunteers. J Clin Pharmacol 40:1338–1346
Sevilla-Tirado FJ, Gonzalez-Vallejo EB, Leary AC et al (2003) Bioavailability of two new formulations of paracetamol, compared with three marketed formulations, in healthy volunteers. Methods Find Exp Clin Pharmacol 25:531–535
Podilsky G, Berger-Gryllaki M, Testa B et al (2008) Development and validation of an HPLC method for the simultaneous monitoring of bromazepam and omeprazole. J Liq Chrom Rel Technol 31:878–890
Lacey LF, Keene ON, Pritchard JF et al (1997) Common noncompartmental pharmacokinetic variables: are they normally or log-normally distributed? J Biopharm Stat 7:171–178
Henderson AR (2006) Testing experimental data for univariate normality. Clin Chim Acta 366:112–129
Weisstein EW (2006) Quantile-quantile plot. In: MathWorld. Wolfram Web Resource. Wolfram Research, Champaign, IL. Available at: http://mathworld.wolfram.com/Quantile-QuantilePlot.html. Accessed 10 Nov 2006
Weisstein EW (2006) Distribution Function. In: MathWorld. Wolfram Web Resource. Wolfram Research, Champaign, IL. Available at: http://mathworld.wolfram.com/DistributionFunction.html. Accessed 20 Oct 2006
Huguier M, Flahault A (2000) Biostatistiques au Quotidien. Elsevier, Paris
European Agency for the Evaluation of Medicinal Products (EMEA) Committee for Proprietary Medicinal Products (2001) Note for Guidance on the Investigation of Bioavailability and Bioequivalence. In: Scientific guidelines for human medical products. Available at: http://www.emea.europa.eu/pdfs/human/ewp/140198en.pdf. Accessed 10 Feb 2008
U.S. Food and Drug Administration (FDA). Center for Drug Evaluation and Research (CDER) (2001) Statistical approaches to establishing bioequivalence. In: Guidance for industry. FDA, Washington D.C. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=320. Accessed 17 Feb 2008
R Development Core Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at: http://www.R-project.org. Accessed Mar 2006
Sostek MB, Chen Y, Skammer W et al (2003) Esomeprazole administered through a nasogastric tube provides bioavailability similar to oral dosing. Aliment Pharmacol Ther 18:581–586
Dunn A, White CM, Reddy P (1999) Delivery of omeprazole and lansoprazole granules through a nasogastric tube in vitro. Am J Health Syst Pharm 56:2327–2330
Acknowledgements
The authors express their gratitude to Ms. Monique Appenzeller for her help during the trials, the nutrition nurse Ms. Isabelle Bordier, the technicians in the bioanalytical laboratory and the volunteers in this trial.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Podilsky, G., Berger-Gryllaki, M., Testa, B. et al. The bioavailability of bromazepam, omeprazole and paracetamol given by nasogastric feeding tube. Eur J Clin Pharmacol 65, 435–442 (2009). https://doi.org/10.1007/s00228-008-0613-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0613-4