Abstract
Objective
This study was designed to characterize the population pharmacokinetics of pyrazinamide in South African pulmonary tuberculosis patients, with special reference to interindividual and interoccasional variability (IIV and IOV, respectively).
Methods
Concentration-time measurements obtained from 227 patients receiving oral doses of pyrazinamide were pooled to create a dataset containing 3,092 data points spanning multiple dosing occasions. The software program NONMEM was used to analyze the data.
Results
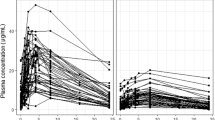
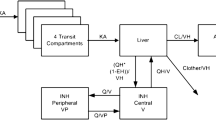
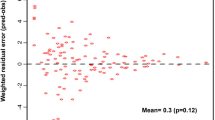
A one-compartment model with first-order absorption, including a zero-order component describing release from formulation, and first-order elimination best described the data. The absorption rate constant was estimated to be bimodally distributed between two distinct subgroups, fast and slow, in approximately even proportion. Absorption rate was threefold greater in fast absorbers (3.56 h−1) in comparison to slow absorbers (1.25 h−1). Typical values of oral clearance and apparent volume of distribution were estimated as 3.42 L h−1 and 29.2 l, respectively. IOV was supported in oral clearance (0.0238, variance) and absorption rate (0.623, variance). The duration of zero-order absorption was estimated as 0.290 h, and was quite variable between patients (0.957, variance).
Conclusion
The absorption of pyrazinamide in the studied population was highly variable and two separate subpopulations were identified. IOV accounted for a proportion of the variability in clearance and the absorption rate constant.




Similar content being viewed by others
References
Agrawal S, Panchagnula R (2005) Implication of biopharmaceutics and pharmacokinetics of rifampicin in variable bioavailability from solid oral dosage forms. Biopharm Drug Dispos 26:321–334
Beal SL, Sheiner LB, Boeckmann A (1996) NONMEM Users’ Guides. NONMEM Project Group, University of California, San Francisco
Ellard GA (1969) Absorption, metabolism and excretion of pyrazinamide in man. Tubercle 50:144–158
Hausler H (2000) Tuberculosis & HIV/AIDS: Clinical Guidelines. National Department of Health, South Africa
Heifets L, Lindholm-Levy P (1992) Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am Rev Respir Dis 145:1223–1225
Jonsson EN, Karlsson MO (1999) Xpose-an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64
Konno K, Feldmann FM, McDermott W (1967) Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis 95:461–469
Maher D, Mikulencak M (1999) What is DOTS? A guide to understanding the WHO-recommended TB control strategy known as DOTS. WHO/CDS/CPC/TB/99.270. World Health Organization, Geneva
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm 20:511–528
McDermott W, Tompsett R (1954) Activation of pyrazinamide and nicotinamide in acidic environment in vitro. Am Rev Tuberc 70:748–754
Mitchison DA (2000) Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis 4:796–806
Peloquin CA, Jaresko GS, Yong CL, Keung AC, Bulpitt AE, Jelliffe RW (1997) Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother 41:2670–2679
Salfinger M, Heifets LB (1988) Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob Agents Chemother 32:1002–1004
Shishoo CJ, Shah SA, Rathod IS, Savale SS, Vora MJ (2001) Impaired bioavailability of rifampicin in presence of isoniazid from fixed dose combination (FDC) formulation. Int J Pharm 228:53–67
Singh S, Mariappan TT, Sankar R, Sarda N, Singh B (2001) A critical review of the probable reasons for the poor variable bioavailability of rifampicin from anti-tubercular fixed-dose combination (FDC) products, and the likely solutions to the problem. Int J Pharm 228:5–17
Smith PJ, van Dyk J, Fredericks A (1999) Determination of rifampicin, isoniazid and pyrazinamide by high performance liquid chromatography after their simultaneous extraction from plasma. Int J Tuberc Lung Dis 3:S325–S328
Tarshis MS, Weed WA (1953) Lack of significant in vitro sensitivity of Mycobacterium tuberculosis to pyrazinamide on three different solid media. Am Rev Tuberc 67:391–395
The National Essential Drugs List Committee (1998) Standard treatment guidelines and essential drugs list for South Africa: adults: hospital level. National Department of Health, Pretoria
Wählby U, Jonsson EN, Karlsson MO (2001) Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 28:231–252
Wilkins JJ (2005) NONMEMory: a run management tool for NONMEM. Comput Methods Programs Biomed 78:259–267
World Health Organization (2003) Treatment of Tuberculosis: Guidelines for National Programmes. World Health Organization, Geneva
Yeager RL, Munroe WG, Dessau FI (1952) Pyrazinamide (aldinamide) in the treatment of pulmonary tuberculosis. Am Rev Tuberc 65:523–546
Zhang Y, Scorpio A, Nikaido H, Sun Z (1999) Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Bacteriol 181:2044–2049
Zhu M, Starke JR, Burman WJ, Steiner P, Stambaugh JJ, Ashkin D, Bulpitt AE, Berning SE, Peloquin CA (2002) Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy 22:686–695
Acknowledgements
We thank Jean van Dyk, Rudy Onia, Afia Fredericks and Alicia Evans for their invaluable technical and logistical assistance. We also wish to thank Bernard Fourie for allowing the use of some of the data included in this study. This research was co-funded by the South African Medical Research Council and by the Division of Clinical Pharmacology of the Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa. All work described here was carried out with full written ethical approval from the University of Cape Town, Brewelskloof Hospital and the D P Marais SANTA Centre, and complies fully with South African legal requirements for biomedical research.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00228-006-0178-z
Rights and permissions
About this article
Cite this article
Wilkins, J.J., Langdon, G., McIlleron, H. et al. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur J Clin Pharmacol 62, 727–735 (2006). https://doi.org/10.1007/s00228-006-0141-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0141-z