Abstract
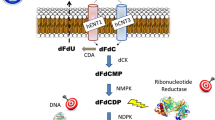
Some anticancer agents tend to accumulate during repeated administration. We determined whether gemcitabine or its metabolites would accumulate during repeated administration. Gemcitabine was administered over two courses with each course consisting of a 30-min infusion at 1000 mg/m2 weekly for 3 weeks followed by 1 week of rest. In 14 patients we evaluated eventual accumulation by comparing the concentrations in blood samples taken before, and at 30 and 60 min after the start of infusion on days 1, 8 and 15, in both cycles. At the end of the infusion gemcitabine concentrations at day 1 of both courses varied between 18 and 77 μM and at day 15 between 13 and 90 μM. The mean ratios day 8/day 1 and day 15/day 1 varied from 0.94 to 1.18. For the inactive metabolite 2',2'–difluoro–2'–deoxyuridine (dFdU) these values varied between 54 and 152 μM and 55 and 157, respectively, and the ratios from 0.96 to 1.08. The concentration of the active metabolite of gemcitabine, gemcitabine triphosphate (dFdCTP) in peripheral white blood cells, ranged between 37 and 283 pmol/106 cells at the end of infusion on day 1 and 35 and 115 pmol/106 cells on day 15. Potential accumulation was evaluated using a mixed effects model and no evidence was observed of accumulation for either gemcitabine or its metabolites. Gemcitabine can be administered safely without the risk that the drug will accumulate.
Similar content being viewed by others
References
Abratt RP, Sandler A, Crino L et al (1998) Combined cisplatin and gemcitabine for non-small cell lung cancer: influence of scheduling on toxicity and drug delivery. Semin Oncol 25:35–43
Bergman AM, Pinedo HM, Peters GJ (2002) Determinants of resistance to 2',2'–difluorodeoxycytidine (gemcitabine). Drug Res Updates 5:19–33
Borner MM, Schöffski P, De Wit R et al (2002) Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomized crossover trial in advanced colorectal cancer. Eur J Cancer 38:349–358
Boublil JL, Milano G, Khater R et al (1985) Continuous 5-day regional chemotherapy by 5–fluorouracil in colon carcinoma: pharmacokinetic evaluation. Br J Cancer 52:15–20
Brown H, Prescott R (1999) Applied mixed models in medicine. Wiley, Chichester, UK
Burris HA, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: a randomized trial. J Clin Oncol 15:2403–2413
Casper ES, Green MR, Kelsen DP et al (1994) Phase II trial of gemcitabine (2',2'–difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs 12:29–34
Csapo Z, Keszler G, Sasvari–Szekely M et al (1998) Similar changes were induced by Cladribine and by gemcitabine, in the deoxypyrimidine salvage, during short-term treatments. Adv Exp Med Biol 431:525–529
Grunewald R, Kantarjian H, Keating MJ et al (1990) Pharmacologically directed design of the dose rate and schedule of 2',2'–difluorodeoxycytidine (Gemcitabine) administration in leukemia. Cancer Res 50:6823–6826
Grunewald R, Kantarjian H, Du M et al (1992) Gemcitabine in leukemia: a phase I clinical, plasma, and cellular pharmacology study. J Clin Oncol 10:406–413
Huang P, Plunkett W (1992) A quantitative assay for fragmented DNA in apoptotic cells. Anal Biochem 207:163–167
Humerickhouse RA, Dolan ME, Haraf DJ et al (1999) Phase I study of eniluracil, a dihydropyrimidine dehydrogenase inactivator, and oral 5–fluorouracil with radiation therapy in patients with recurrent or advanced head and neck cancer. Clin Cancer Res 5:291–298
Kroep JR, Peters GJ. Van Moorsel CJA et al (1999) Gemcitabine–cisplatin: a schedule finding study. Ann Oncol 10:1503–1510
Kroep JR, Giaccone G, Voorn DA et al (1999) Gemcitabine and paclitaxel; pharmacokinetic and pharmacodynamic interactions in patients with non-small cell lung cancer. J Clin Oncol 17:2190–2197
Lippe P, Silva RR, Giuliodori L et al (2002) Clinical benefit of gemcitabine–cisplatin in advanced non-small cell lung cancer elderly patients. Anticancer Res 22:1053–1059
National Cancer Institute (1998) Guidelines for reporting adverse reactions. Division of cancer treatment, National Cancer Institute, Bethesda, MD
Plunkett W, Huang P, Searcy CE et al (1996) Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol 23:3–15
Ruiz van Haperen VWT, Veerman G, Vermorken JB et al (1993) 2',2'–Difluoro–deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochem Pharmacol 46:762–766
Ruiz van Haperen VWT, Veerman G, Boven E et al (1994) Schedule dependence of sensitivity to 2',2'–difluorodeoxycytidine (gemcitabine) in relation to accumulation and retention of its triphosphate in solid tumor cell lines and solid tumors. Biochem Pharmacol 48:1327–1339
Storniolo MA, Allerheiligen SRB, Pearce HL (1997) Preclinical, pharmacologic, and Phase I studies of gemcitabine. Semin Oncol 24 (suppl 7):2–7
Van Moorsel CJA, Peters GJ, Pinedo HM (1997) Gemcitabine: future prospects of single-agent and combination studies. The Oncologist 2:127–134
Van Moorsel CJA, Kroep JR, Pinedo HM et al (1999) Pharmacokinetic schedule finding study of the combination of gemcitabine and cisplatin in patients with solid tumors. Ann Oncol 10:441–448
Acknowledgments
This study was sponsored by Eli Lilly International, UK. The study was approved by the Ethical Committees of both institutes in the Netherlands and Denmark, and performed according to the local laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Lange, S.M., van der Born, K., Kroep, J.R. et al. No evidence of gemcitabine accumulation during weekly administration. Eur J Clin Pharmacol 61, 843–849 (2005). https://doi.org/10.1007/s00228-005-0033-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0033-7