Abstract
Effective seabird conservation requires understanding their marine spatial ecology. Tracking can reveal details of their foraging ecology and habitat use, as well as the suitability of marine protected areas for at-sea conservation, but results are often regionally specific. Here we characterised the foraging behaviour of tropical breeding brown boobies Sula leucogaster in the Chagos Archipelago, Western Indian Ocean, and tested habitat requirements. GPS tracking of thirteen individuals from two colonies, located 142 km apart on the same atoll (Great Chagos Bank), showed similar foraging effort and habitat preferences despite differences in season and breeding stage. Brown boobies from both tracked populations foraged close to the colony along the atoll shelf edge, avoiding deep oceanic areas and shallow waters of the Great Chagos Bank atoll, but within the Chagos Archipelago Marine Protected Area. Sea-level height anomaly and sea surface temperature were important foraging predictors at both sites, although birds experienced distinct environmental conditions between colonies. These results suggest that while brown boobies have colony-specific at-sea foraging areas, similarities in habitat drivers of distribution and foraging behaviour can inform predictions of distributions at other colonies within the archipelago, with important benefits for at-sea conservation efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studying seabird foraging ecology is important not only because it explains their extreme life histories (Dobson and Jouventin 2007), but also to understand their key role as ecosystem engineers (Graham et al. 2018; Otero et al. 2018), and their value as bioindicators of prey abundance and marine productivity (Bost et al. 2008; Velarde et al. 2013). However, seabird populations are under intense pressure on land and at-sea, making them one of the most threatened groups of birds worldwide (Croxall et al. 2012; Dias et al. 2019).
The establishment of Marine Protected Areas (MPAs) and Birdlife International’s Important Bird and Biodiversity Areas (IBAs) have become two primary methods for the conservation of seabird foraging habitats. Studies on seabird distributions, foraging behaviour, and habitat use are common approaches for identifying potential MPA locations (Grecian et al. 2012; Lascelles et al. 2012; Le Corre et al. 2012; Thaxter et al. 2012). The creation of large-scale MPAs has surged in recent years with the aim to protect wide-ranging species, including seabirds, but are still constrained in their effectiveness due to various management challenges (O’Leary et al. 2018). Advances in seabird tracking technologies have promoted greater and more comprehensive scientific understanding of species behaviours at sea, which has in turn aided in both the establishment of MPAs (Wakefield et al. 2009; Hays et al. 2019) and the evaluation of their effectiveness (Harris et al. 2007). These behaviours, which are informed by measures such as trip duration and distance, dive depth, and habitat use, are broadly referred to as foraging strategies. Furthermore, data collected from tracking devices can reveal utilisation distributions, indicating the probability density of an animal’s location at any given time (Powell and Mitchell 2012). Nevertheless, both foraging strategies and utilisation distributions can vary considerably within and among species, making the identification of foraging hotspots for conservation prioritisation, including MPA designation, more challenging.
Behavioural variation within and among species is central to ecology, and has implications for scaling of conservation measures (Brakes and Dall 2016; Cleasby et al. 2023). For example, for breeding seabirds, central-place foraging strategies can scale among colonies at local scales (Warwick-Evans et al. 2018). However, variability in behaviour is common both within- (Votier et al. 2017; Trevail et al. 2021) and among colonies at regional scales (> 200 km; Mannocci et al. 2017; Miller et al. 2023; Cleasby et al. 2023). Divergent foraging strategies often arise through local adaptation to environmental conditions such as wind (Raymond et al. 2010; Nourani et al. 2023), and oceanographic features such as bathymetry, sea surface temperature, and heterogeneity (e.g., Weimerskirch et al. 2005; Waggitt et al. 2018; Trevail et al. 2019), and can therefore vary both spatially and temporally (Mendez et al. 2017). Lastly, foraging strategies are dependent on interactions with other marine foragers; in tropical regions, sub-surface predators drive prey closer to the surface in reach of flying predators (Au and Pitman 1986; Vaughn et al. 2008; Correia et al. 2019), meanwhile resource competition can drive segregation within and among species (Almeida et al. 2021; Trevail et al. 2023). These sources of variation in individual and population behaviour could limit the ability to infer priority sites for at-sea protection from limited tracking data (Soanes et al. 2013). As such, understanding foraging strategies among populations is a key step for maximising use of existing tracking data and guiding future studies in conservation and management.
The Chagos Archipelago is a group of five above-surface atolls and home to some of the world’s most pristine coral reefs (Sheppard et al. 2012). It was declared a no-take MPA in April 2010, covering circa 640,000 km2 of the Western Indian Ocean. The MPA was established to protect marine biodiversity, including seabirds, their breeding and foraging habitats (Sheppard et al. 2012). However, because of its remoteness, access limitations, and expanse, seabird research has been limited. The brown booby, Sula leucogaster, is a pantropical oceanic species and one of the 18 breeding seabird species found in the Chagos Archipelago (Carr et al. 2021b). Brown boobies’ diet mostly consists of pelagic fish (Harrison et al. 1984; Mellink et al. 2001) but can be variable in response to environmental conditions (Castillo-Guerrero et al. 2016). Foraging trip duration for the species is short compared to more pelagic sulid species (Lewis et al. 2004; Weimerskirch et al. 2009; Soanes et al. 2015). Furthermore, there is evidence of sex differences in foraging behaviour of brown boobies, larger females often undertake longer foraging trips and can forage at different trophic levels (Lewis et al. 2005; Weimerskirch et al. 2009; Young et al. 2010; Miller et al. 2018). Foraging areas may also vary depending on season and breeding stage; seabirds typically travel further during incubation (Soanes et al. 2015). Whilst previous studies have highlighted how foraging strategies vary for the species within colonies (Weimerskirch et al. 2009; Miller et al. 2018; Austin et al. 2021) and when compared with colonies in different oceans (Soanes et al. 2015, 2016; Correia et al. 2021), there is limited evidence for foraging variability among colonies within in the same archipelago or atoll (Soanes et al. 2016). Understanding inter-colony variations in the same geographic areas can be used to better address the establishment and assess the effectiveness of MPAs and IBAs.
This study aims to characterise brown booby foraging strategies for colonies located in the same archipelago. Breeding brown boobies from two islands situated 142 km apart on the Great Chagos Bank atoll, in the Chagos Archipelago, were fitted with GPS loggers to document foraging trip metrics and at-sea behaviours, and explore foraging habitat use. Because of logistical constraints on fieldwork, birds were tracked during different years, monsoon seasons, and breeding stages. Because these differences are known to influence foraging strategies, inter-colony variability is expected.
Materials and methods
Study site
Fieldwork was conducted on Nelson’s Island (72.3126° E, 5.6815° S) in July 2018 and Danger Island (71.2406° E, 6.3869° S) in January 2019 (Table 1). These two remote, uninhabited islands are respectively situated in the North and West of the Great Chagos Bank, the largest living coral atoll in the world (Sheppard et al. 2012). Brown boobies breed at three major colonies in the archipelago; North Brother (max 740 pairs), Danger Island (max 141 pairs, 35 recorded during study period), and Nelson’s Island (max 35 pairs, 12 during study period) (Carr et al. 2021b). Elsewhere in the Chagos Archipelago, colonies comprising fewer than 8 breeding pairs have been recorded across 5 islands (Carr et al. 2021b). Danger Island and Nelson’s Island are therefore important within the archipelago, although populations are not large enough meet IBA qualifying criteria for regional significance (Carr et al. 2021b). Coordinates of the Chagos Archipelago MPA were downloaded from the World Database on Protected Areas, managed by UNEP World Conservation Monitoring Centre (available at ProtectedPlanet.net).
GPS deployment and retrieval
To study at-sea foraging behaviour, 21 breeding adult brown boobies were fitted with a unique British Trust of Ornithology (BTO) metal leg ring, livestock marker to aid recapture, an archival GPS logger (15 g, IGotU GT-120, Mobile Action Technology Inc) attached to the tail with tesa tape, and a leg-mounted light level geolocator (3 g, Intigeo C330, Migrate Technology—data not used in this study). Handling time per individual was approximately 5–6 min. Together, GPS loggers and geolocators weighed < 3% of the individual’s body mass and there were no detectable effects of device deployment and recovery on breeding success during each expedition. On both Danger and Nelson’s Island we monitored all brown booby nests where tags were deployed, and a selection of nests where no adults were caught, and at the point when we left each island none of the nests were observed to have failed.
GPS loggers were programmed to obtain fixes every 5 min (24 h a day) and deployed for between 3–8 days. The stage of breeding and nest GPS locations for the tagged individuals were recorded, and when possible, the bird’s sex was identified by morphological differences (Nelson 1978).
Data processing
First, downloaded GPS data were trimmed to incorporate tag deployment and retrieval periods only. A 300 m buffer was then created around the islands and used to exclude short trips, typically for bathing or rafting (Bennison et al. 2018; Trevail et al. 2019). Complete trips were defined as journeys starting and finishing within 300 m of the colony including a minimum of four fixes.
To evaluate the GPS logger performance, GPS trip fix success rate was statistically analysed using a Wilcoxon Signed-Rank Test as observed and expected fix success rate data were non-normal. Despite there being a difference between observed and expected fix success rate (P < 0.05), a frequency histogram (available in the Supplementary Material, Fig. S1) revealed that the majority of trips had only one or zero missing locations, and only three trips contained recording gaps of over an hour of data. Because of this preliminary analysis, and experimental evidence suggesting high performance of this GPS logger (Morris and Conner 2017), performance of loggers was deemed suitable for further analyses. All statistical analyses were conducted in R 4.0.0 (R Core Team 2016).
Foraging trip metrics
GPS fixes from complete trips were used to estimate total trip duration (mins), total trip distance (km), and maximum distance travelled (km), using the R package trip (Sumner et al. 2009).
To test for differences in foraging trips between colonies, trip metrics were square-root transformed to meet assumptions of normality (Young et al. 2015). Data distributions before and after transformation supported use of transformed data for colony comparisons (Supplementary material Fig. S2). Because of the hierarchical nature of the data, inter-colony variations in trip metrics were assessed with ‘individual’ (i.e. bird ID) as a random effect in a linear mixed effect model (LMM) using the function lmer in R package lme4 (Bates et al. 2015). Differences between colonies were assessed by removing colony from the model using the function drop1 in lme4. For further data exploration, correlations between trip metrics were assessed using a Spearman’s rank correlation, selected after running a Shapiro–Wilk test that indicated a non-normal distribution in trip metric data.
At-sea behaviours
At-sea movement behaviour was quantified using a Hidden Markov Model (HMM) in R package moveHMM (Michelot et al. 2016). This time series model is suitable for making inferences on animal movement behaviour through a non-observable state sequence derived from an observable series (Langrock et al. 2012). The model calculates changes in step length (i.e. distance) and turning angle between GPS points to annotate trajectories by behavioural state (Michelot et al. 2016). Firstly, GPS data were linearly interpolated to every 5 min using default parameters in the R package adehabitat (Calenge 2017); this step accounted for missing locations associated with a GPS fix success rate of < 100% and allowed these data to be run using an HMM on equally spaced locations.
The HMM with three states fitted these data better than a two-state mode; (based on Akaike Information Criteria, AIC, Supplementary material Table S1), as is the case for other booby species (Boyd et al. 2014; Oppel et al. 2015; Miller et al. 2018). The Viterbi algorithm was used to estimate the most likely sequence of movement states. The HMM revealed three distinct underlying behavioural states (Fig. S6). A short step length and narrow turning angle (step: 0.07 ± 0.04 km; turn: μ = 0.02, κ = 38) corresponded to a slow-moving state, described in the literature as a ‘resting’ state (Boyd et al. 2014; Miller et al. 2018). A greater step length and the widest turning angle (step: 0.9 ± 0.8 km; turn: μ =− 0.004, κ = 1.5) revealed a ‘foraging’ behaviour. Finally, a ‘transiting’ state was matched to a much greater step length, and the narrowest turning angle (step: 3.2 ± 0.6 km; turn: μ = − 0.01, κ = 19). Transiting would correspond to a relatively straight section of the trajectory in which GPS points are spaced out by an average of 3.2 km (Fig. S6), corresponding to a flight speed of roughly 38 km/h. Visual examination of the HMM output revealed that the proportion of time spent foraging was 100% in some of the shortest trips in distance, hence a Spearman’s rank correlation was run to further investigate a relationship between maximum trip distance from the colony and proportion of time spent in each state. The analyses for this, found in Supplementary Material Fig. S3, suggested that the output for the HMM could be used to examine foraging behaviour of the tracked individuals. To understand inter-colony differences in behavioural states, binomial GLMMs were run on the proportion of time spent in each behaviour, with ‘trip’ and ‘individual’ as nested random effects.
Habitat selection
To understand how brown booby occurrence and foraging locations were linked to environment, we used habitat-selection models based on available locations (i.e., all locations within each individuals’ home range) and known foraging locations determined by the HMM (i.e., locations that were documented by tracking devices) to test if tracked animals show preference towards particular habitats within their foraging range. To sample the available environment, a circle polygon with a radius of the furthest trip from the respective colony was drawn around the two colonies (i.e. 111.5 km around Danger Island and 67 km around Nelson’s Island). Ten GPS points were randomly generated for every foraging GPS point (as indicated by the HMM) in the tracking data within this radius using the function spsample in R package sp (Trevail et al. 2019).
We sampled static and dynamic environmental variables at all used and available points that could influence the availability and accessibility of foraging opportunities for brown boobies; (1) bathymetry, (2) bathymetric slope, (3) sea surface temperature (SST), (4) chlorophyll, (5) sea-level height anomaly (SLA), (6) wind speed, (7) wind direction, and (8) distance to the colony. NOAA ETOPO1 bathymetry (1-arc minute resolution) was downloaded using package marmap (Pante et al. 2023). Slope was obtained from bathymetry using function “terrain” in the R package raster (Hijmans and Etten 2018). Dynamic variables were downloaded using the erddap interface from NOAA oceanwatch (https://oceanwatch.pifsc.noaa.gov/erddap/): sea surface temperature (NOAA geopolar blended—monthly), chlorophyll a concentration (ESA OC CCI—monthly), sea surface height anomalies and geostrophic currents (NOAA/NESDIS/STAR), and ocean surface winds (CCMP, daily). Distance from colony was obtained using function “distGeo” in package geosphere (Hijmans et al. 2022).
For each colony, we ran habitat selection models using the biomod2 package (Thuiller et al. 2009) to predict presence/absence based on environmental variables. To avoid the idiosyncratic effect of different model types we constructed ensemble models comprising random forests (RF), generalised linear models (GLM), generalised additive models (GAM), maximum entropy (MAXENT), boosted regression trees (GBM), and artificial neural networks (ANN). Model performance was evaluated on tenfold cross validation using relative operating characteristic (ROC) and true skills statistic (TSS) scores, where values closer to 1 indicate improved model discrimination between presence and absences (ROC) and presence prediction (TSS). Ensemble performance was evaluated using an 80/20 split. Ensemble predictions used the weighted sum of probabilities (EMwmeanByTSS). We assessed variable importance and made spatial predictions using this ensemble model.
Results
GPS data
Tag recovery success was 73% on Danger Island (11 loggers) and 100% on Nelson’s Island (6 loggers, Table 1), yielding tracking data for nine birds on Danger Island and four on Nelson’s Island (Table 2; battery failure occurred on the remaining loggers). On Danger Island, data were from three males and six females. On Nelson’s Island, data were from two males, and two birds of unknown sex. Because of low sample sizes, no formal comparisons were made between foraging strategies of different sexes. All tracks were located within the Chagos Archipelago MPA (Fig. 1). Individuals from both colonies travelled away from the central Great Chagos Bank into surrounding, deeper waters (Fig. 1).
Trips (n = 74) performed by GPS tagged Brown boobies (n = 13) from two separate colonies (triangles). (a) Trips are coloured by individual and overlaid on bathymetry map. (b) Location of the Chagos Archipelago in the Indian Ocean with MPA boundary shown in green. (c) Tracks from both colonies occur close to the islands, well within the Chagos Archipelago MPA
Foraging trip metrics
We analysed 74 trips from the 13 individuals (Table 2), compising 2–9 trips over 3–8 days per individual. In general, birds departed for one long foraging trip per day or multiple shorter ones, and trips took place during daytime. Across both colonies, trips ranged from 0.3–12.7 h duration, covered a total distance of 2.3–252.8 km, and reached a maximum of 1.1–111.5 km from the colony. On Danger Island, foraging trips lasted, on average, 3.8 ± 0.4 h, reached a maximum of 35.6 ± 3.8 km from the colony, and covered a total distance of 88.0 ± 9.0 km. On Nelson’s Island, foraging trips lasted 3.7 ± 0.5 h, reached a maximum of 23.9 ± 3.7 km from the colony, and covered a total distance of 63.0 ± 9.0 km. There were strong positive correlations between all trip metrics (Figure S4). Trip duration for both sites combined was positively correlated with total trip distance (rs = 0.899, P < 0.001, n = 74) and maximum distance travelled from the nest (rs = 0.829, P < 0.001). Total trip distance and maximum distance travelled were also positively correlated (rs = 0.973, P < 0.001). Although trip metrics were marginally shorter on Nelson’s Island than on Danger Island (Table 2), there was no significant inter-colony variation; trip duration (F1 = 0.08, P = 0.78), total distance (F1 = 1.09, P = 0.31), maximum distance (F1 = 1.93, P = 0.19) (Fig. 2). Trip metrics are presented for individuals by sex in the Supplementary Material (Fig. S5).
At-sea behaviour
On average, tracked individuals (colonies pooled) spent 57% of the time foraging, 38% of the time transiting, and 5% of the time resting. All deconstructed tracks for both colonies and a close-up individual example are respectively found in the Supplementary Material (Figure S7 & S8). Tracked individuals at Nelson’s Island spent a significantly greater proportion of time foraging (GLMM, χ2(1) = 15.4, P < 0.001) and a lower proportion of time transiting (χ2(1) = 488, P < 0.001), whereas there was no statistical difference in the proportion of time resting (χ2(1) = 150, P = 0.53) between colonies (Fig. 3).
Habitat selection
Individual habitat selection models had high performance (cross-validation AUC > 0.85, TSS generally > 0.7 at both colonies; Figure S9). For predictions, we formed ensemble models combining all model types at each colony, with high AUCs (0.98 for Danger Island, 0.985 for Nelson’s Island).
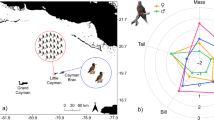
Variable importance assessment (Fig. S10) highlighted that distance to colony was a strong predictor at both colonies, with individuals preferring habitat closer to their colony (Fig. 4) relative to the overall environment available within their foraging range. Sea-level height anomaly and sea-surface temperature were also important at both colonies but with different ranges likely associated with different at-sea conditions (Fig. 4). At both colonies individuals also preferred habitat with steep slopes (Fig. 4) along the shelf edge. Other variables differed in their importance at each colony. Wind-speed was a useful predictor for birds from Danger Island (perhaps as wind speed was consistently higher, Fig. 4); chlorophyll-a and bathymetric depth were important predictors at Nelson (with individuals preferring shallower, less productive waters, Fig. 4). Based on these habitat preferences, we predicted brown boobies from the two tracked colonies to forage nearby to their colonies, in areas extending outwards into deeper waters, away from the central Great Chagos Bank (Fig. 5).
Probability of habitat use along environmental gradients for GPS tracked brown boobies from Danger Island (pink) and Nelson’s Island (blue), based on ensemble models (error bars show confidence intervals based on 100 ensemble iterations). Birds at each colony experienced different ranges of dynamic variables, but all preferentially foraged closer to the colony and at steeper bathymetric slopes
Discussion
Our results reveal similar patterns of at-sea behaviour between two segregated colonies of brown boobies in the Chagos Archipelago. Despite differences in colony location, monsoon period, year, and breeding stage during tracking, trip metrics did not differ significantly between colonies. Foraging similarities could arise because of shared environmental preferences for shelf edge habitats close to the colony. Both colonies foraged in distinct at-sea areas inside the MPA.
Brown boobies used comparable foraging strategies at both tracked colonies in the Chagos Archipelago with similar trip lengths of < 12.7 h duration (Fig. 2), foraged at distal locations (Figure S7) up to a maximum of 111.5 km from the colony, and spent very little time resting at sea (Fig. 3). Nevertheless, we note that the number of tracked individuals is quite small (DI n = 9, NI n = 4) and while similarities should be treated with caution, all tracked birds had more than one trip (Table 1). Trip durations and distances were similar to those reported for this species in other oceans (Table 3), further highlighting that brown boobies generally forage in neritic zones and along coastal shelf edges (Weimerskirch et al. 2009; Young et al. 2015; Miller et al. 2018; Correia et al. 2021; Austin et al. 2021). However, given that temporal changes in the environment can influence foraging strategies (Soanes et al. 2015), and seabirds often forage further from the colony during incubation (Sommerfeld and Hennicke 2010), it is interesting that variability in foraging effort was not observed between study colonies, perhaps because of shared environmental preferences.
Brown boobies are one of three sulids breeding in the Chagos Archipelago, alongside red-footed boobies, Sula sula (total 22,871 pairs), and masked boobies, Sula dactylatra (total 164 pairs) (Carr et al. 2021b). Brown boobies forage much closer to the islands than the smaller, more pelagic red-footed boobies (mean maximum distance: 112.9 ± 3.7 in Chagos Archipelago, (Trevail et al. 2023), that are resident in the archipelago year-round (Votier et al. 2024). Masked booby at-sea distributions in the Chagos Archipelago are unknown, however population sizes are smaller, so intraspecific competition is less likely.
The habitat selection analyses demonstrated that distance to the colony was the most important environmental predictor across both populations, followed by sea-level height anomaly and sea-surface temperature. Although the range of these dynamic variables differed across the two tracking periods, birds at both colonies foraged in areas with higher sea-level height anomaly than available, indicative of eddy circulation and potential nutrient entrainment (Hyrenbach et al. 2006). Sea-surface temperature was also an important driver of habitat selection at both colonies, with birds at Nelson’s Island selecting intermediate temperatures compared to those available between 27.5 and 28 °C, and birds at Danger Island selecting warmer waters > 29.5 °C (Fig. 4). Both of these temperature ranges coincide with favourable feeding habitats of skipjack tuna (Druon et al. 2017), so could suggest preference for facultative feeding opportunities, although further investigation into foraging modality is necessary (Dunn et al. 2024). At both colonies, individuals foraged over steeper slopes, away from the central Great Chagos Bank, similar to brown boobies in the Great Barrier Reef that also forage on the reef edge (Miller et al. 2018). Bathymetric slopes are associated with greater productivity as currents and topography can drive prey closer to the surface (Piatt et al. 2006; Reisinger et al. 2018), thereby attracting foraging seabirds (Piatt et al. 2006; McDuie et al. 2018; De Pascalis et al. 2020).
There were significant differences in the proportion of time spent in foraging and transiting states between the colonies. Differences may be explained by trip distances (Fig. S3); birds on Danger Island travelled marginally greater distances than birds from Nelson’s Island, and therefore may have incurred greater transit distances (Oppel et al. 2015). Alternatively, birds at Nelson’s Island experienced greater wind speeds (Fig. 4), potentially facilitating lower transit times. Female brown boobies sometimes travel further than males during foraging trips (Lewis et al. 2005; Weimerskirch et al. 2009; Soanes et al. 2015; Bunce 2015; Miller et al. 2018), although the reverse may also occur (Austin et al. 2021). Sample sizes here precluded formal testing, although we note no obvious sex differences in descriptive statistics (Fig. S5).
Foraging effort and at-sea distribution models are both used to help designate MPAs for seabirds (Lascelles et al. 2012; Le Corre et al. 2012). Our results demonstrate the value of the Chagos Archipelago large scale MPA for encompassing the foraging area and habitat preferences of brown boobies during breeding. This is encouraging for seabirds that appear to be increasing following the long-term impacts of invasive rats and vegetation (Carr et al. 2021a, b). Nevertheless, distribution of brown boobies within the Chagos Archipelago MPA does not imply complete protection of foraging resources, as illegal fishing remains a concern within the MPA (Collins et al. 2021).
Here, we attempted to understand variability in foraging strategies of two colonies of brown boobies in the Chagos Archipelago. As outlined previously, limited access to field sites meant that individuals between the two colonies were tracked during different years, monsoon periods, and breeding stages, and that sample sizes of both sexes were relatively low. Whilst similarities in foraging strategies are therefore noteworthy, future studies in the region could focus on filling these data gaps and providing greater understanding of intra-colony variability in this species.
Because of low sample sizes, at-sea tracks (Fig. 1) may under-represent true population distributions (Soanes et al. 2013). Despite the limited number of individuals with retrieved tracking data, incorporating multiple trips per individual undoubtedly improves estimation of area use (Soanes et al. 2013). Understanding the proportion of general and core foraging areas represented by tracked individuals is dependent on multiple factors, including year, and sex. Therefore, drawing conclusions on the effectiveness of the Chagos Archipelago MPA surrounding Danger Island and Nelson’s Island using brown boobies could be assessed in future studies with a particular emphasis on sample sizes.
Conclusion
To conclude, we found comparable foraging strategies by brown boobies from nearby islands (142 km apart) in the Chagos Archipelago despite differences in tracking periods and breeding stage. Brown boobies foraged in coastal areas along the shelf-edge, which contrasts the more pelagic behaviour of neighbouring red-footed boobies (Trevail et al. 2023), but is consistent with studies of this species elsewhere (Austin et al. 2021). Importantly, we found that foraging boobies avoided the shallow water of the Grand Chagos Bank atoll, but showed a preference for areas with a steeper bathymetric slope, higher sea-surface height anomalies, and ranges of SST that correspond to tuna foraging habitat. Finally, brown boobies remained within the Chagos Archipelago MPA suggesting that this large-scale MPA can offer protection to habitats and prey.
Data availability
The datasets generated and analysed in this study are available on the BirdLife Seabird Tracking Database at www.seabirdtracking.org data set ID’s 1685 and 1686.
References
Almeida N, Ramos JA, Rodrigues I, dos Santos I, Pereira JM, Matos DM, Araújo PM, Geraldes P, Melo T, Paiva VH (2021) Year-round at-sea distribution and trophic resources partitioning between two sympatric Sulids in the tropical Atlantic. PLoS ONE 16:e0253095. https://doi.org/10.1371/JOURNAL.PONE.0253095
Au DWK, Pitman RL (1986) Seabird interactions with dolphins and tuna in the eastern tropical pacific. Condor 88:304–317. https://doi.org/10.2307/1368877
Austin RE, De Pascalis F, Votier SC, Haakonsson J, Arnould JPY, Ebanks-Petrie G, Newton J, Harvey J, Green JA (2021) Interspecific and intraspecific foraging differentiation of neighbouring tropical seabirds. Mov Ecol 9:1–16. https://doi.org/10.1186/S40462-021-00251-Z/TABLES/2
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bennison A, Bearhop S, Bodey TW, Votier SC, Grecian WJ, Wakefield ED, Hamer KC, Jessopp M (2018) Search and foraging behaviors from movement data: a comparison of methods. Ecol Evol 8:13–24. https://doi.org/10.1002/ece3.3593
Boyd C, Punt AE, Weimerskirch H, Bertrand S (2014) Movement models provide insights into variation in the foraging effort of central place foragers. Ecol Model 286:13–25. https://doi.org/10.1016/j.ecolmodel.2014.03.015
Brakes P, Dall SRX (2016) Marine mammal behavior: a review of conservation implications. Front Mar Sci. https://doi.org/10.3389/fmars.2016.00087
Bunce A (2015) Foraging behaviour of a declining population of Brown Boobies ( Sula leucogaster ) breeding in the Swain Reefs, Great Barrier Reef. Emu Austr Ornithol 115:368–372. https://doi.org/10.1071/MU14037
Calenge C (2017) adehabitatHR: home range estimation. 46
Carr P, Trevail A, Bárrios S, Clubbe C, Freeman R, Koldewey HJ, Votier SC, Wilkinson T, Nicoll MAC (2021a) Potential benefits to breeding seabirds of converting abandoned coconut plantations to native habitats after invasive predator eradication. Restor Ecol 29:e13386. https://doi.org/10.1111/rec.13386
Carr P, Votier S, Koldewey H, Godley B, Wood H, Nicoll MAC (2021b) Status and phenology of breeding seabirds and a review of important bird and biodiversity areas in the British Indian ocean territory. Bird Conservation International 31:14–34. https://doi.org/10.1017/S0959270920000295
Castillo-Guerrero JA, Lerma M, Mellink E, Suazo-Guillén E, Peñaloza-Padilla EA (2016) Environmentally-mediated flexible foraging strategies in brown boobies in the Gulf of California. Ardea 104:33–47. https://doi.org/10.5253/arde.v104i1.a3
Cleasby IR, Owen E, Butler A, Baer J, Blackburn J, Bogdanova MI, Coledale T, Daunt F, Dodd S, Evans JC, Green JA, Guilford T, Harris MP, Hughes R, Newell MA, Newton SF, Robertson GS, Ruffino L, Shoji A, Soanes LM, Votier SC, Wakefield ED, Wanless S, Wilson LJ, Bolton M (2023) Assessing the importance of individual- and colony-level variation when using seabird foraging ranges as impact assessment and conservation tools. Ibis Ibi. https://doi.org/10.1111/ibi.13284
Collins C, Nuno A, Broderick A, Curnick DJ, de Vos A, Franklin T, Jacoby DMP, Mees C, Moir-Clark J, Pearce J, Letessier TB (2021) Understanding persistent non-compliance in a remote, large-scale marine protected area. Front Mar Sci 8:650276. https://doi.org/10.3389/fmars.2021.650276
Correia E, Granadeiro J, Mata V, Regalla A, Catry P (2019) Trophic interactions between migratory seabirds, predatory fishes and small pelagics in coastal West Africa. Mar Ecol Prog Ser 622:177–189. https://doi.org/10.3354/meps13022
Correia E, Catry P, Sinclair F, Dos Santos Y, Robalo JI, Lima CS, Granadeiro JP (2021) Foraging behaviour and diet of Brown boobies Sula leucogaster from Tinhosas Islands. Gulf of Guinea Mar Biol 168:91. https://doi.org/10.1007/s00227-021-03904-0
De Pascalis F, Imperio S, Benvenuti A, Catoni C, Rubolini D, Cecere JG (2020) Sex-specific foraging behaviour is affected by wind conditions in a sexually size dimorphic seabird. Anim Behav 166:207–218. https://doi.org/10.1016/j.anbehav.2020.05.014
Dobson FS, Jouventin P (2007) How slow breeding can be selected in seabirds: testing Lack’s hypothesis. Proc R Soc B Biol Sci 274:275–279. https://doi.org/10.1098/rspb.2006.3724
Druon J-N, Chassot E, Murua H, Lopez J (2017) Skipjack tuna availability for purse seine fisheries is driven by suitable feeding habitat dynamics in the Atlantic and Indian oceans. Front Mar Sci 4:315. https://doi.org/10.3389/fmars.2017.00315
Dunn RE, Freeman R, Nicoll MA, Ramsden J, Trevail AM, Wood H, Votier SC (2024) From route to dive: multi-scale habitat selection in a foraging tropical seabird. Mar Biol 171:124. https://doi.org/10.1007/s00227-024-04445-y
Graham NAJ, Wilson SK, Carr P, Hoey AS, Jennings S, MacNeil MA (2018) Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559:250–253. https://doi.org/10.1038/s41586-018-0202-3
Grecian WJ, Witt MJ, Attrill MJ, Bearhop S, Godley BJ, Grémillet D, Hamer KC, Votier SC (2012) A novel projection technique to identify important at-sea areas for seabird conservation: an example using Northern gannets breeding in the North East Atlantic. Biol Cons 156:43–52. https://doi.org/10.1016/j.biocon.2011.12.010
Harris J, Haward M, Jabour J, Woehler EJ (2007) A new approach to selecting marine protected areas (MPAs) in the Southern Ocean. Antarct Sci 19:189–194. https://doi.org/10.1017/S0954102007000260
Harrison CS, Hida TS, Seki MP (1984) The diet of the brown booby Sula leucogaster and Masked Booby Sula dactylatra on Rose Atoll, Samoa. Ibis 126:588–590. https://doi.org/10.1111/j.1474-919X.1984.tb02082.x
Hays GC, Bailey H, Bograd SJ, Bowen WD, Campagna C, Carmichael RH, Casale P, Chiaradia A, Costa DP, Cuevas E, Nico de Bruyn PJ, Dias MP, Duarte CM, Dunn DC, Dutton PH, Esteban N, Friedlaender A, Goetz KT, Godley BJ, Halpin PN, Hamann M, Hammerschlag N, Harcourt R, Harrison AL, Hazen EL, Heupel MR, Hoyt E, Humphries NE, Kot CY, Lea JSE, Marsh H, Maxwell SM, McMahon CR, Notarbartolo di Sciara G, Palacios DM, Phillips RA, Righton D, Schofield G, Seminoff JA, Simpfendorfer CA, Sims DW, Takahashi A, Tetley MJ, Thums M, Trathan PN, Villegas-Amtmann S, Wells RS, Whiting SD, Wildermann NE, Sequeira AMM (2019) Translating marine animal tracking data into conservation policy and management. Trends Ecol Evol 34:459–473. https://doi.org/10.1016/j.tree.2019.01.009
Hijmans RJ, van Etten J (2018) raster: geographic analysis and modeling with raster data. R Package Version 2(7–15):244
Hijmans RJ, Karney C, Williams E, Vennes C (2022) Spherical trigonometry for geographic applications. That is, compute distances and related measures for angular (longitude/latitude) locations
Hyrenbach KD, Veit RR, Weimerskirch H, Hunt GL (2006) Seabird associations with mesoscale eddies: the subtropical Indian Ocean. Mar Ecol Prog Ser 324:271–279. https://doi.org/10.3354/meps324271
Lascelles BG, Langham GM, Ronconi RA, Reid JB (2012) From hotspots to site protection: identifying marine protected areas for seabirds around the globe. Biol Cons 156:5–14. https://doi.org/10.1016/j.biocon.2011.12.008
Le Corre M, Jaeger A, Pinet P, Kappes MA, Weimerskirch H, Catry T, Ramos JA, Russell JC, Shah N, Jaquemet S (2012) Tracking seabirds to identify potential marine protected areas in the tropical western Indian Ocean. Biol Cons 156:83–93. https://doi.org/10.1016/j.biocon.2011.11.015
Lewis S, Schreiber EA, Daunt F, Schenk GA, Wanless S, Hamer KC (2004) Flexible foraging patterns under different time constraints in tropical boobies. Anim Behav 68:1331–1337. https://doi.org/10.1016/J.ANBEHAV.2004.04.007
Lewis S, Schreiber EA, Daunt F, Schenk GA, Orr K, Adams A, Wanless S, Hamer KC (2005) Sex-specific foraging behaviour in tropical boobies: does size matter? Ibis 147:408–414. https://doi.org/10.1111/J.1474-919X.2005.00428.X
Mannocci L, Boustany AM, Roberts JJ, Palacios DM, Dunn DC, Halpin PN, Viehman S, Moxley J, Cleary J, Bailey H, Bograd SJ, Becker EA, Gardner B, Hartog JR, Hazen EL, Ferguson MC, Forney KA, Kinlan BP, Oliver MJ, Perretti CT, Ridoux V, Teo SLH, Winship AJ (2017) Temporal resolutions in species distribution models of highly mobile marine animals: recommendations for ecologists and managers. Divers Distrib 23:1098–1109. https://doi.org/10.1111/ddi.12609
McDuie F, Weeks SJ, Congdon BC (2018) Oceanographic drivers of near-colony seabird foraging site use in tropical marine systems. Mar Ecol Prog Ser 589:209–225. https://doi.org/10.3354/meps12475
Mellink E, Domínguez J, Luévano J (2001) Diet of Eastern Pacific Brown Boobies Sula leucogaster brewsteri on Isla San Jorge, north-eastern Gulf of California, and an April comparison with diets in the middle Gulf of California. Mar Ornithol 29:23–28
Mendez L, Borsa P, Cruz S, De Grissac S, Hennicke J, Lallemand J, Prudor A, Weimerskirch H, Grissac SD, Hennicke J, Lallemand J, Prudor A, Weimerskirch H (2017) Geographical variation in the foraging behaviour of the pantropical red-footed booby. Mar Ecol Prog Ser 568:217–230. https://doi.org/10.3354/meps12052
Michelot T, Langrock R, Patterson TA (2016) moveHMM: an R package for the statistical modelling of animal movement data using hidden Markov models. Methods Ecol Evol 7:1308–1315. https://doi.org/10.1111/2041-210X.12578
Miller MGR, Silva FRO, Machovsky-Capuska GE, Congdon BC (2018) Sexual segregation in tropical seabirds: drivers of sex-specific foraging in the Brown Booby Sula leucogaster. J Ornithol 159:425–437. https://doi.org/10.1007/S10336-017-1512-1/TABLES/4
Miller M, Hemson G, Toit JD, McDougall A, Miller P, Mizutani A, Trevail A, Small A, Ravache A, Beard A, Bunce A, Poli C, Surman C, Gonzalez-Zamora D, Clingham E, Vidal E, McDuie F, Machovsky-Capuska G, Cumming G, Humphries G, Weimerskirch H, Shamoun-Baranes J, Henry L, Wood H, Young H, Kohno H, Gonzalez-Sols J, Cecere J, Veen J, Neumann J, Shephard J, Green J, Castillo-Guerrero J, Sommerfeld J, Dossa J, Bourgeois K, Yoda K, McLeay L, Calabrese L, Mendez L, Soanes L, Nicoll M, Derhé M, Gilmour M, Diop N, James N, Carr P, Austin R, Freeman R, Clarke R, Mott R, Maxwell S, Saldanha S, Shaffer S, Oppel S, Votier S, Yamamoto T, Militão T, Beger M, Congdon B (2023) Refining seabird marine protected areas by predicting habitat inside foraging range a case study from the global tropics. Preprints
Morris G, Conner LM (2017) Assessment of accuracy, fix success rate, and use of estimated horizontal position error (EHPE) to filter inaccurate data collected by a common commercially available GPS logger. PLoS ONE 12:e0189020. https://doi.org/10.1371/journal.pone.0189020
Nelson JB (1978) The Sulidae, Gannets and Boobies. Oxford University Press, Oxford
Nourani E, Safi K, De Grissac S, Anderson DJ, Cole NC, Fell A, Grémillet D, Lempidakis E, Lerma M, McKee JL, Pichegru L, Provost P, Rattenborg NC, Ryan PG, Santos CD, Schoombie S, Tatayah V, Weimerskirch H, Wikelski M, Shepard ELC (2023) Seabird morphology determines operational wind speeds, tolerable maxima, and responses to extremes. Curr Biol 33:1179-1184.e3. https://doi.org/10.1016/j.cub.2023.01.068
O’Leary BC, Ban NC, Fernandez M, Friedlander AM, García-Borboroglu P, Golbuu Y, Guidetti P, Harris JM, Hawkins JP, Langlois T, McCauley DJ, Pikitch EK, Richmond RH, Roberts CM (2018) Addressing criticisms of large-scale marine protected areas. Bioscience 68:359–370. https://doi.org/10.1093/biosci/biy021
Oppel S, Beard A, Fox D, Mackley E, Leat E, Henry L, Clingham E, Fowler N, Sim J, Sommerfeld J, Weber N, Weber S, Bolton M (2015) Foraging distribution of a tropical seabird supports Ashmole’s hypothesis of population regulation. Behav Ecol Sociobiol 69:915–926
Otero XL, De La Peña-Lastra S, Pérez-Alberti A, Ferreira TO, Huerta-Diaz MA (2018) Seabird colonies as important global drivers in the nitrogen and phosphorus cycles. Nat Commun 9:1–8. https://doi.org/10.1038/s41467-017-02446-8
Pante E, Simon-Bouhet B, Irisson J-O (2023) marmap: import, plot and analyze bathymetric and topographic data.
Piatt JF, Wetzel J, Bell K, DeGange AR, Balogh GR, Drew GS, Geernaert T, Ladd C, Byrd GV (2006) Predictable hotspots and foraging habitat of the endangered short-tailed albatross (Phoebastria albatrus) in the North Pacific: implications for conservation. Deep Sea Res Part II 53:387–398. https://doi.org/10.1016/j.dsr2.2006.01.008
Powell RA, Mitchell MS (2012) What is a home range? J Mammal 93:948–958. https://doi.org/10.1644/11-MAMM-S-177.1
R Core Team (2016) R: A language and environment for statistical computing.
Raymond B, Shaffer SA, Sokolov S, Woehler EJ, Costa DP, Einoder L, Hindell M, Hosie G, Pinkerton M, Sagar PM, Scott D, Smith A, Thompson DR, Vertigan C, Weimerskirch H (2010) Shearwater foraging in the southern ocean: the roles of prey availability and winds. PLoS ONE 5:e10960. https://doi.org/10.1371/journal.pone.0010960
Reisinger RR, Raymond B, Hindell MA, Bester MN, Crawford RJM, Davies D, de Bruyn PJN, Dilley BJ, Kirkman SP, Makhado AB, Ryan PG, Schoombie S, Stevens K, Sumner MD, Tosh CA, Wege M, Whitehead TO, Wotherspoon S, Pistorius PA (2018) Habitat modelling of tracking data from multiple marine predators identifies important areas in the Southern Indian Ocean. Divers Distrib 24:535–550. https://doi.org/10.1111/ddi.12702
Sheppard CRC, Ateweberhan M, Bowen BW, Carr P, Chen CA, Clubbe C, Craig MT, Ebinghaus R, Eble J, Fitzsimmons N, Gaither MR, Gan C, Gollock M, Guzman N, Graham NAJ, Harris A, Jones R, Keshavmurthy S, Koldewey H, Lundin CG, Mortimer JA, Obura D, Pfeiffer M, Price ARG, Purkis S, Raines P, Readman JW, Riegl B, Rogers A, Schleyer M, Seaward MRD, Sheppard ALS, Tamelander J, Turner JR, Visram S, Vogler C, Vogt S, Wolschke H, Yang JM, Yang S, Yesson C (2012) Reefs and islands of the Chagos Archipelago, Indian Ocean: why it is the world’s largest no-take marine protected area. Aquat Conserv 22:232–261. https://doi.org/10.1002/aqc.1248
Soanes LM, Arnould JPY, Dodd SG, Sumner MD, Green JA (2013) How many seabirds do we need to track to define home-range area? J Appl Ecol 50:671–679. https://doi.org/10.1111/1365-2664.12069
Soanes LM, Bright JA, Bolton M, Millett J, Mukhida F, Green JA (2015) Foraging behaviour of brown boobies Sula leucogaster in Anguilla, Lesser Antilles: preliminary identification of at-sea distribution using a time-in-area approach. Bird Conserv Int 25:87–96. https://doi.org/10.1017/S095927091400001X
Soanes LM, Bright JA, Carter D, Dias MP, Fleming T, Gumbs K, Hughes G, Mukhida F, Green JA (2016) Important foraging areas of seabirds from Anguilla, Caribbean: implications for marine spatial planning. Mar Policy 70:85–92. https://doi.org/10.1016/j.marpol.2016.04.019
Sommerfeld J, Hennicke JC (2010) Comparison of trip duration, activity pattern and diving behaviour by Red-tailed Tropicbirds (Phaethon rubricauda) during incubation and chick-rearing. Emu 110:78–86. https://doi.org/10.1071/MU09053
Sumner MD, Wotherspoon SJ, Hindell MA (2009) Bayesian estimation of animal movement from archival and satellite tags. PLoS ONE. https://doi.org/10.1371/journal.pone.0007324
Thaxter CB, Lascelles B, Sugar K, Cook ASCP, Roos S, Bolton M, Langston RHW, Burton NHK (2012) Seabird foraging ranges as a preliminary tool for identifying candidate Marine Protected Areas. Biol Cons 156:53–61
Thuiller W, Lafourcade B, Engler R, Araújo MB (2009) BIOMOD—a platform for ensemble forecasting of species distributions. Ecography 32:369–373. https://doi.org/10.1111/J.1600-0587.2008.05742.X
Trevail AM, Green JA, Sharples J, Polton JA, Arnould JPY, Patrick SC (2019) Environmental heterogeneity amplifies behavioural response to a temporal cycle. Oikos 128:517–528. https://doi.org/10.1111/oik.05579
Trevail AM, Green JA, Bolton M, Daunt F, Harris SM, Miller PI, Newton S, Owen E, Polton JA, Robertson G, Sharples J, Patrick SC (2021) Environmental heterogeneity promotes individual specialisation in habitat selection in a widely distributed seabird. J Anim Ecol 90:2875–2887. https://doi.org/10.1111/1365-2656.13588
Trevail AM, Wood H, Carr P, Dunn R, Nicoll M, Votier S, Freeman R (2023) Multi-colony tracking reveals segregation in foraging range, space use, and timing in a tropical seabird. Mar Ecol Prog Ser 724:155–165. https://doi.org/10.3354/meps14479
Vaughn RL, Würsig B, Shelton DS, Timm LL, Watson LA (2008) Dusky dolphins influence prey accessibility for seabirds in Admiralty Bay, New Zealand. J Mammal 89:1051–1058. https://doi.org/10.1644/07-MAMM-A-145.1
Votier SC, Fayet AL, Bearhop S, Bodey TW, Clark BL, Grecian J, Guilford T, Hamer KC, Jeglinski JWE, Morgan G, Wakefield E, Patrick SC (2017) Effects of age and reproductive status on individual foraging site fidelity in a long-lived marine predator. Proc R Soc B Biol Sci 284:20171068. https://doi.org/10.1098/rspb.2017.1068
Votier SC, Corcoran G, Carr P, Dunn RE, Freeman R, Nicoll MAC, Wood H, Trevail AM (2024) Geolocation and immersion loggers reveal year-round residency and facilitate nutrient deposition rate estimation of adult red-footed boobies in the Chagos Archipelago, tropical Indian Ocean. J Avian Biol. https://doi.org/10.1111/jav.03185
Waggitt JJ, Cazenave PW, Howarth LM, Evans PGH, van der Kooij J, Hiddink JG (2018) Combined measurements of prey availability explain habitat selection in foraging seabirds. Biol Let 14:20180348. https://doi.org/10.1098/rsbl.2018.0348
Wakefield ED, Phillips RA, Matthiopoulos J (2009) Quantifying habitat use and preferences of pelagic seabirds using individual movement data: a review. Mar Ecol Prog Ser 391:165–182. https://doi.org/10.3354/meps08203
Warwick-Evans V, Ratcliffe N, Lowther AD, Manco F, Ireland L, Clewlow HL, Trathan PN (2018) Using habitat models for chinstrap penguins Pygoscelis antarctica to advise krill fisheries management during the penguin breeding season. Divers Distrib 24:1756–1771. https://doi.org/10.1111/DDI.12817
Weimerskirch H, Le Corre M, Jaquemet S, Marsac F (2005) Foraging strategy of a tropical seabird, the red-footed booby, in a dynamic marine environment. Mar Ecol Prog Ser 288:251–261. https://doi.org/10.3354/meps288251
Weimerskirch H, Shaffer S, Tremblay Y, Costa D, Gadenne H, Kato A, Ropert-Coudert Y, Sato K, Aurioles D (2009) Species- and sex-specific differences in foraging behaviour and foraging zones in blue-footed and brown boobies in the Gulf of California. Mar Ecol Prog Ser 391:267–278. https://doi.org/10.3354/meps07981
Young H, McCauley D, Dirzo R, Dunbar R, Shaffer S (2010) Niche partitioning among and within sympatric tropical seabirds revealed by stable isotope analysis. Mar Ecol Prog Ser 416:285–294. https://doi.org/10.3354/meps08756
Young HS, Maxwell SM, Conners MG, Shaffer SA (2015) Pelagic marine protected areas protect foraging habitat for multiple breeding seabirds in the central Pacific. Biol Cons 181:226–235. https://doi.org/10.1016/j.biocon.2014.10.027
Acknowledgements
The research was funded by the Bertarelli Foundation as part of the Bertarelli Programme in Marine Science. The authors would also like to thank the reviewers of this manuscript.
Funding
Funding for this research was provided by the Bertarelli Foundation (BPMS 2017-5).
Author information
Authors and Affiliations
Contributions
Data collection was performed by Peter Carr, Hannah Wood and Malcolm Nicoll and data analysis was conducted by Sonia Vallocchia, Alice Trevail, Robin Freeman, and Malcolm Nicoll. The first draft of the manuscript was written by Sonia Vallocchia. Subsequent drafts were led by Alice Trevail. All authors contributed to editing and preparing this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The capture, handling and tagging of Brown boobies was approved by the Special Marks Panel of the British Trust for Ornithology and the Zoological Society of London’s Ethical Committee.
Additional information
Responsible Editor: T. Clay.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trevail, A.M., Vallocchia, S., Nicoll, M.A.C. et al. Comparable foraging effort and habitat use between two geographically proximate tropical seabird colonies. Mar Biol 171, 165 (2024). https://doi.org/10.1007/s00227-024-04464-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04464-9