Abstract
Many shark and ray species show affinity to specific sites, making these areas critical for their survival. These include cleaning stations: locations on reefs where cleaner fish remove parasites and clean wounds, which is important for maintaining health. Cleaning stations also function as social gathering sites, or resting points, where courtship and mating can occur. In this study, we identify an aggregation site for the shortfin devil ray, Mobula kuhlii (Family Mobulidae) within the Aliwal Shoal Marine Protected Area in KwaZulu-Natal (KZN), South Africa, and document their behavior. Remote underwater video was used to collect footage of M. kuhlii being cleaned by blue streak cleaner wrasse, Labroides dimidiatus. Generalized additive models (GAMs) were used to assess environmental predictors of M. kuhlii presence on Aliwal Shoal. Mixed models were used to assess the same environmental predictors and their correlation with mean M. kuhlii cleaning duration and number of L. dimidiatus bites per second at the identified cleaning station site. M. kuhlii were present in 56% of observation days, with group numbers up to > 150 individuals. Sea surface temperature was a significant predictor for M. kuhlii presence, while a north to south current was significantly associated with longer mean cleaning durations. These results support findings of mobulid studies in KZN that show increased habitat use during summer temperatures (24–25 °C) and suggest these sites to be important for individual health and social interaction. We hope these findings can be used for development of location-specific management plans to safeguard this Endangered species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine cleaning stations are areas of reef where a client (teleost, shark, ray, turtle) visits to have parasites or dead tissue removed by cleaner fish (Limbaugh 1961; Feder 1966; Losey 1972) or shrimps and have been studied for over 70 years. Keyes (1982) first described shark cleaning behavior in an aquarium, observing Atlantic lemon sharks (Negaprion brevirostris), Pacific lemon sharks (N. acutidens), a bull shark (Carcharhinus leucas), sandbar sharks (C. plumbeus), and nurse sharks (Ginglymostoma cirratum) being cleaned by the bluestreak cleaner wrasse Labroides dimidiatus. Subsequently, this behavior has been documented in free-living elasmobranch species at various locations (Table S1). Most shark and ray (Elasmobranchii) species likely benefit from cleaning services, but only a handful of such species have been observed being cleaned in the wild.
Elasmobranchs are ubiquitously infected by metazoan parasites, such as gnathid isopods and caligid copepods, which can lead to lesions, necrosis, anemia, and respiratory diseases (Caira and Healy 2004). Cleaning behavior can alleviate these effects, and has been linked to better body condition in some fish species when compared to conspecifics who did not have access to cleaning stations (Ros et al. 2020). In addition to providing essential health services, cleaning stations function as social aggregation and courtship/mating grounds for elasmobranchs (Oliver and Kaszo 2015; Stevens 2016; Perryman et al. 2019, 2022a). Individuals or groups of clients repeatedly return to cleaning stations seasonally or periodically throughout the year, often displaying long-term affinity to specific sites (Oliver et al. 2011; Germanov et al. 2019; Perryman et al. 2019). Thus, these locations can play a crucial role in the health and reproduction, of many elasmobranch species, serving as habitats to which they consistently return.
The family Mobulidae (Mobula spp.) are pelagic/epipelagic, migratory species found circumglobally in tropical, subtropical, and temperate seas (Notarbartolo di Sciara 1987; Stewart et al. 2018). Mobulid species have K-selected reproductive strategies, reproducing by aplacental vivipary, with late maturation and the lowest reported fecundity among elasmobranchs (Dulvy et al. 2014), making them highly vulnerable to exploitation and incidental capture (Couturier et al. 2012; Croll et al. 2016). All mobulids are listed as Vulnerable or Endangered on the IUCN Red List of threatened species as a result of directed capture for the gill plate trade in Asia and incidental capture as bycatch by industrial trawlers, gill nets, seine nets, and longlines throughout their distribution (Croll et al. 2016). Mobula kuhlii is listed as Endangered (Rigby et al. 2022) with declines in sighting records of up to 99% being observed in Tofo, Mozambique (Rohner et al. 2017). However, little is known about this species in South Africa, which is the southernmost limit of its range in the Western Indian Ocean (Rigby et al. 2022).
Although known to be heavily parasitized by caligid copepods in KwaZulu-Natal (KZN), South Africa (Lebepe and Dippenaar 2013), the only reported occurrence of M. kuhlii at cleaning stations is from the Bazaruto Archipelago, Mozambique (Murie and Marshall 2016) and no such sites are known from South Africa. Other mobulid species including manta rays have been found to inhabit the KZN coastline from Richard’s Bay in the north southwards to Port Edward, with significantly more presence in summer and in the area encompassing Aliwal Shoal (Carpenter et al. 2023). Many different mobulid species have been caught in the area (Young 2001). Apart from one study that documented first-time courtship behavior of M. kuhlii at Aliwal Shoal (Carpenter and Griffiths 2023), no research on this species has been conducted in the country to date.
In this study we document sightings, habitat use, and behavior of M. kuhlii in the Aliwal Shoal Marine Protected Area (MPA), in KZN. This includes the discovery and description of a cleaning station area for this species. As mobulid habitat use is known to be influenced by environmental cues, we used Generalized Additive Models to assess 1) environmental effects (sea surface temperature, current) on the presence of M. kuhlii on Aliwal Shoal and 2) cleaning durations on Angels Ledge (AL) cleaning station. Using Remote Underwater Video (RUV) we describe interspecific cleaning behavior and interactions with cleaner fish. The results are informative for understanding fine-scale habitat use of this understudied species.
Materials and methods
Study area
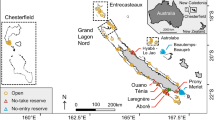
The Aliwal Shoal Marine Protected Area (MPA), is a subtropical sponge and algal reef located four km offshore from the mKomzai River Mouth in KwaZulu-Natal Province (KZN), South Africa (Olbers et al. 2009) (Fig. 1). The reef is influenced by the dominant south-flowing Agulhas Current, which brings warm, nutrient-poor water from the Mozambique Channel; as well as by wind-driven eddies and subsequent upwellings which bring in nutrient rich and productive waters (Heydorn et al. 1978; Hutchings et al. 2002). Aliwal Shoal is renowned for its biodiversity, and specifically, as a site for encounters with rare and threatened sharks, such as the ragged tooth shark (Carcharias taurus) and tiger shark (Galeocerdo cuvier) (Dicken et al. 2006; Dicken and Hosking 2009). An area covering 18.3 km of coastline and 126 km2 of ocean was designated an MPA in 2004, to prevent anchoring and mooring of vessels, and extraction of marine resources (Marine Living Resources Act No. 18 of 1998, Government Gazette No. 26433, South Africa 2004). The study area was in the Crown Area Restricted Zone, which is approximately 1 km long, and 280–890 m wide, encompassing an area of 2.1 km2 (Bosman et al. 2005), with an average depth of 12.5 m, but reaching as shallow as 6 m (Bosman et al. 2007).
Data collection
Sightings logbook
Data were collected from opportunistic recreational SCUBA or snorkeling trips between September 2020 and March 2022. Eleven sites varying in depth (6–26 m) and in exposure to swell and wave action within the Crown Area Restricted Zone in Aliwal Shoal (Fig. 1; Table S2) were surveyed for M. kuhlii cleaning stations. Due to the close proximity of the sites to one another and the frequent currents that occur at Aliwal Shoal, multiple named sites were often visited during a single survey.
A single recreational ‘dive’, or a recreational snorkel ‘drift’, was defined as a single drift over the reef from one location to another, going with the current. The names of the sites and the times spent at each were recorded in situ using an underwater slate and underwater dive computer. The time spent at each location was measured as effort, with minute (min) being the unit of effort. The maximum number of individuals of M. kuhlii (Max N) present at the same time, and their behaviors, were recorded during each dive. Sightings per unit effort (SPUE) were then calculated for each site. Environmental conditions recorded were current direction (N, S, E, W, or ‘none’), wind speed (km/h), wind direction (°), sea surface temperature (SST; 1 °C intervals), bottom sea temperature (BST; 1 °C intervals), and estimated horizontal visibility (m). Temperature was measured using an underwater dive computer. Daily moon phase data were sourced from the ‘suncalc’ package (Thieurmel and Elmarhraoui 2019) in R Studio (R Core Team 2021).
‘Cleaning’ behavior was defined as observation of a cleaner fish making obvious contact with the body of one or more M. kuhlii. The observation of cleaning behavior at a specific location in at least two survey days resulted in the location being designated a cleaning station. A surface marker buoy signaled the skipper on the surface to mark the precise coordinates of such sites on a Lowrance Elite 5 GPS system.
Collecting footage of Mobula kuhlii cleaning behavior
Due to frequent observations of cleaning behavior during preliminary surveys, one cleaning station (Angels Ledge (AL), Figure S1), at a depth of 22 m, was selected for more detailed observation of M. kuhlii cleaning behavior. Remote underwater video (RUV) was obtained by placing a GoPro Hero 5 or Hero 9 attached to two 1 kg dive weights in the sand facing the ledge by freediving or during recreational SCUBA dives. Two RUVs were placed for one hour at a time, back-to-back to ensure no overlap in field of view, et al. (Figure S1). These then gathered video data of M. kuhlii cleaning behavior in the absence of humans.
Evaluating/processing data
Coding behavior and cleaning interactions
Videos were processed using frame by frame analysis in BORIS Software (Friard and Gamba 2016), whereby user-defined behaviors could be permanently logged, as point or continuous observations, showing their frequencies and durations. When M. kuhlii were present in the video the maximum number (Max N) of individuals present in one frame was recorded. Behavior data could not be collected on separate M. kuhlii individuals due to the inability to identify them by unique spot patterning as it is currently unknown how to distinguish individuals of this species. The sex of each individual, maturity status, and present and type of injures were recorded, where possible. Sex was determined by presence or absence of claspers, with fully extended claspers signifying a mature male, and the presence of mating scars or pregnancy indicating mature females (Notarbartolo di Sciara 1987; Marshall and Bennett 2010a; White et al. 2006). Injuries were identified by crescent-shaped scars, which were attributed to predation (Marshall and Bennett 2010b), or triangular scars and/or truncated tail injuries, which were attributed to either predation or entanglement in monofilament (Deakos et al. 2011; Germanov et al. 2019). Sex ratio and injury prevalence were calculated based only on individuals that were close enough to the camera during video recording to describe these.
Cleaning interactions were defined as any cleaner fish making contact with (‘biting’) a M. kuhlii, indicating attempted parasite removal (Oliver et al. 2011; Murie et al. 2020). Cleaning time was defined as periods of time where cleaner fish were surrounding M. kuhlii individuals, or within 1 m of them, but not necessarily biting the whole time. This allowed for determination of the cleaning effort (average bites per second of cleaning time). Several aspects of cleaning behavior were recorded, including ‘hovering or swimming slow’ (Fig. 2a), ‘jolting’ (Fig. 2b), when a client suddenly jerks part or all of its body, possibly in response to ‘cheating’ cleaner fish (Soares et al. 2008) or uncomfortable interactions, ‘posing’ (Fig. 2c) which involves terminating pectoral fin beats and opening the mouth and gills (O’Shea et al. 2010), and ‘following’ (Fig. 2d) which involves mobulid individuals following each other around a cleaning station (Perryman et al. 2021).
‘Cruising’ behavior was recorded when M. kuhlii were swimming in one direction, either singly or in a group, with the cephalic lobes furled and clearly not engaged in cleaning (Table 1). If individuals were observed in the area and exhibiting aspects of cleaning behavior, but were too far away to detect cleaner fish bites, this was recorded as ‘cleaning out of sight’.
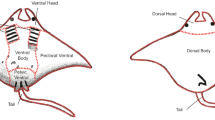
As different areas of a client’s body are known to host different parasites (Bshary and Grutter 2002; Caira and Healy 2004; Marshall 2008; Oliver et al. 2011; Murie et al. 2020), both the number of bites and the region of the body being cleaned were recorded. The same eight body patches outlined by Murie et al. (2020) were used to evaluate the specific areas of M. kuhlii cleaned by a cleaner fish, these being: tail, gills, pelvic fins (ventral), ventral body cavity, ventral pectoral fins, ventral head, dorsal head, and dorsal body (Figure S2). Cleaner interactions were recorded in BORIS software using a point-event function named ‘Cleaner fish bite’, with body part selected as a modifier. Cephalic lobe positions were also recorded when they changed during a cleaning interaction (both unfurled, both furled, one unfurled and one furled) to compare with manta ray cephalic lobe use at cleaning stations (Perryman et al. 2021).
Cleaning species have been suggested to compete with one another and to prefer larger over smaller clients (Kitchen-Wheeler 2013). Therefore, the presence of other species that had a total length or disc width of 1 m or greater simultaneously cleaning on AL, such as spotted eagle rays Aetobatus ocellatus, pickhandle barracuda Sphyraena jello, round ribbontail rays Taeniurops meyeni, potato groupers Epinephelus tukula, bull rays Aetomylaeus bovinus, or giant trevally Caranx ignobilis, were also recorded as observations in BORIS. Interactions with these species were then compared with M. kuhlii cleaning durations and cleaner fish bite numbers to determine effects of multiple species using the same cleaning station.
Statistical analysis
Mobula kuhlii presence on aliwal shoal
Generalized additive models (GAMs) fitted with binomial error distributions were used to investigate the effects of environmental conditions on the presence or absence (‘probability of encounter’) of M. kuhlii on the Aliwal Shoal MPA. We fitted GAMs using the packages ‘mgcv’ and ‘nlme’ (Wood 2006) in R software (R Core Team 2021). Data were used from one snorkel drift or dive in a single day, to avoid double counting of encountered M. kuhlii. A stepwise analysis was used to test effects of all variables recorded during a recreational dive or snorkel drift. Moon phase was included with a cyclical smoother and current direction as a categorical variable (Wood 2006). Sequential F-tests were used to determine the covariates that were significant (p < 0.05) to the deviance explained, with non-contributing variables removed from the final model. A Chi square analysis comparison of all models was used to select the best fitted model. The model used to predict M. kuhlii presence on Aliwal Shoal MPA was as follows:
where logit denotes the binomial link function, p is the likelihood of encountering at least one individual, α is the intercept, and s1 signifies a standard smoothing function for SST (Wood 2006).
Cleaning interactions
From the remote underwater video (RUV) cameras, basic statistics on behavior were produced and exported using the ‘synthetic time budget’ code in BORIS software (Friard and Gamba 2016). From this, mean durations of behaviors were calculated (± Standard Deviation). To test the effects of the same environmental parameters tested for M. kuhlii presence, as well as the presence of other megafauna cleaning at the same cleaning station, on mean M. kuhlii cleaning duration (s), a mixed model with gamma distribution was used, with day as a random effect, to account for the lack of independence of many of the remote videos. Mean cleaning duration (s) and number of bites per second (bites / s) were both included in the model sets. These were used instead of total cleaning duration (s), to avoid bias in the abundance or total amount of rays present on the cleaning station. The videos collected by RUVs, were placed at 22 m and were consistently in waters at least 1 degree less than the surface temperature, as displayed by the underwater dive computer. Therefore, Bottom Sea Temperature (BST; °C), instead of SST, was used to account for thermoclines that may affect cleaning visitation. Current direction was treated as a categorical variable. Sequential F-tests were used to determine the covariates that were significant (p < 0.05) to the deviance explained, with non-contributing variables removed from the final model list. Due to a lower sample size from the RUVs, the predictors were assessed individually. The final selected model for testing environmental predictors on mean M. kuhlii cleaning behavior duration on AL was:
The final model for testing environmental predictors and mean cleaning duration (s) on mean L. dimidiatus bites per second on M. kuhlii during cleaning was:
Gamma regression models were fit using stat_smooth function in package ggplot2 (Wickham 2016).
We compared the average number of bites per body patch by L. dimidiatus during cleaning behavior and plotted this. To further elucidate the specific body patches preferred by L. dimidiatus of cleaner fish preferences, pairwise comparisons between different body parts were conducted with a Kruskal–Wallis test and Dunn test with Bonferroni correction for multiple comparisons (Dunn 1961), in R software which was used in all statistics (R Core Team 2021). The z-test was used to describe the relationship to the mean group of values in order to confirm L. dimidiatus preference of one M. kuhlii body patch over another.
Results
Mobula kuhlii visitation to Aliwal Shoal
A total of 329 recreational dives/snorkeling drifts by a single diver were made across 144 days between September 2020-March 2022. From these, Mobula kuhlii were observed to be present 81 times. Sea surface temperature was the only significant predictor of M. kuhlii presence, with probability of occurrence peaking between 24–25 °C (Fig. 3). This model was chosen based on highest significance when compared to other models that included the other predictor variables (edf = 2.346; Ref.df = 2.718; Chi.sq = 10.670; p = 0.007).
Cleaning stations were observed on all dive sites on Aliwal Shoal, with clients including teleost fish, sea turtles, and shark and ray species. M. kuhlii were only observed cleaning in the area of Angels Ledge (Figure S1), Bay of Caves, and Kyles Reef (Fig. 1; Table S2). The only cleaner fish identified cleaning M. kuhlii on these stations was the blue streak cleaner wrasse, Labroides dimidiatus (Figure S3). During cleaning an individual M. kuhlii would swim slowly, terminate pectoral fin beats by hovering over the cleaning station, or exhibit posing behavior, with pelvic fins angled towards the reef, exposing more of the ventral surface. The number of M. kuhlii per minute of diving (mean = 6 ± 10) was highest at Angels Ledge and Kyles Reef (Fig. 4; Table S2), which are adjacent to one another (roughly 30 m apart).
When observed, a group of M. kuhlii consisted of an average of 12 (± 10) individuals. The largest group observed was > 150 individuals, including at least 10–20 individuals RAM feeding, seen on North Sands on 11 December 2021. Other large groups (between 50–75 M. kuhlii) were encountered at Pinnacles, South Sands, AL, and Kyles Reef. When M. kuhlii were encountered during recreational dives or drifts, the most common behavior was cruising (n = 36), followed by cleaning (n = 25), courtship (n = 6), feeding (n = 6), and following spotted eagle rays Aetobatus ocellatus (n = 2), with unknown behavior recorded 7% of the time (n = 6). Breaching behavior was also observed (n = 5). Mobula kuhlii were present across all temperatures recorded, ranging from 19–27 °C, and in all types of current direction, including the absence of current. However, East or West currents were uncommon (1%). During the study, the estimated horizontal visibility ranged from 6–30 m, cloud cover from 5–100%, wind speed between 0–33 km/hr, and moon phase between 0.005–0.993.
Cleaning behavior
Mobula kuhlii cleaning behavior was described in detail for the first time (Table 1). Remote GoPro mounts were placed in the same spot in the sand in the Angels Ledge cleaning station area during 41 recreational dives between January 2021-March 2022. Mobula kuhlii were recorded in 46% (n = 19) of the videos. Between one and 23 (± 7) individuals were observed cleaning at the same time. Up to five L. dimidiatus individuals surrounded a single M. kuhlii simultaneously (Figure S3).
A total of 4.04 h (14,571 secs) of M. kuhlii cleaning behavior was recorded at Angels Ledge. From this, 1.40 h (5,041 secs) of video was of sufficient quality to analyze specific L. dimidiatus bites on specific body parts, types of cleaning behaviors and cephalic lobe positions. A total of 1.18 h (4311 secs) of cruising behavior were recorded. Cleaning behavior had a mean duration of 53.06 secs (± 73.81 s SD) with M. kuhlii receiving direct cleaning bites from L. dimidiatus an average of 31.16 (± 47.71 SD) times, with an overall cleaning rate of 0.59 bites/s while cleaning. The most common type of cleaning behavior was ‘hovering’, with a mean duration of 32.04 s (± 46.54 SD), followed by ‘following’ (20.46 s ± 40.41 SD), ‘posing’ (4.61 s ± 12.61 SD), and ‘jolting’ (3.15 s ± 5.14 SD). Mobula kuhlii was more likely to clean together with other individuals (total = 4,395 s) than alone (total = 645.99 s).
Mobula kuhlii used a variety of cephalic lobe positions (Figure S4). While cleaning, the cephalic lobes were most-commonly both in a furled position (61%; mean = 5.11 times ± 7.40 SD), followed by both being unfurled (20%; mean = 1.63 times ± 2.97 SD), and one unfurled and one furled (19%; mean = 1.56 times ± 2.60). Cephalic lobe positions were changed at a mean of 0.16 times/s (± 0.15 SD position changes/s). Mobula kuhlii cephalic lobes were often unfurled simultaneously as one or more L. dimidiatus approached their face.
Sex could not be determined for most of the M. kuhlii individuals in the video footage, but of those successfully sexed (n = 30), more were males (n = 22) than females (n = 8), with all males being mature (n = 22), and most females of undetermined maturity and only two confirmed as mature (Figure S5). The most prevalent injuries observed were truncated tails (42), scarred pectoral fin (16), complete loss of tail (4), and bent tail (1) (Figure S6). Individuals with no signs of scarring or injury were observed 17 times, but most M. kuhlii observed were too far from the GoPro camera to confidently describe injuries, so 70 individuals were assigned with unknown injuries. Mobula kuhlii was found to clean simultaneously alongside one or more spotted eagle rays, Aetobatus ocellatus (total time = 207.47 s) (Figure S7), followed by pickhandle barracuda, Sphyraena jello (127.73 s), potato groupers, Epinephelus tukula (22.76 s), and loggerhead turtle, Carretta caretta (3.00 s).
Cleaning duration
Mean cleaning duration (s) of M. kuhlii was significantly correlated with current direction et al. cleaning station (Table 2), with longer cleaning times during currents flowing from north to south (Fig. 5). No other predictors significantly affected mean cleaning duration (Table 2).
Mean cleaning duration (s) of Mobula kuhlii at Aliwal Shoal Marine Protected Area, KwaZulu-Natal, South Africa, under different current conditions (lack of current, or ‘none’, current flowing from the north, ‘north’, and current flowing from the south, ‘south’. The thick black lines represent medians, boxes encompass the inter-quartile ranges, whiskers extend to the most extreme data points within 1.5 × the interquartile range outside the box, and the dots show data points beyond the whiskers
The number of L. dimidiatus bites per second on M. kuhlii while cleaning was significantly correlated with current, bottom sea temperature (BST), and mean cleaning duration (Table 3). Currents flowing south to north (South current) resulted in higher bite rates compared to north to south (North current) (Fig. 6), the opposite of the results of mean cleaning duration. This is because higher bites per second rates were significantly correlated with shorter cleaning durations (Fig. 6). Lower bottom temperatures had significantly higher bites per second, with the highest rates generally occurring at 22 °C (Fig. 6).
Cleaner fish bites/s under different environmental conditions, showing variables found to be significant in Table 3: (a) Current direction; (b) Bottom temperature; (c) Mean cleaning duration. Panels (b) and (c) show gamma regression models fit using stat_smooth function in ggplot2
Mobula kuhlii body patch preference by Labroides dimidiatus
There was a significant difference in M. kuhlii body patch foraging preference by L. dimidiatus (Kruskal–Wallis, p < 0.05). The Bonferroni multiple comparison further supported significant associations or differences among body patch groups (Table 4). More L. dimidiatus bites occurred on the dorsal body (significantly higher than dorsal head, gills and tail), and ventral head (significantly more than tail and gills) of M. kuhlii (Table 4). The pelvic fins of M. kuhlii received significantly more L. dimidiatus bites than the tail, gills, and dorsal head (Table 4). The ventral surface of the head, the dorsal surface of the body, and the pelvic fins body patches of M. kuhlii received the most L. dimidiatus bites on average in a given observation (Fig. 7).
Boxplot with logscale of cleaner fish bites by Labroides dimidiatus (y-axis) across the different body patches of Mobula kuhlii (x-axis) during cleaning behaviour at the Aliwal Shoal Marine Protected Area, KwaZulu-Natal, South Africa between 2020–2022. The thick black lines represent medians, boxes encompass the inter-quartile ranges, whiskers extend to the most extreme data points within 1.5 × the interquartile range outside the box, and the dots show data points beyond the whiskers
Discussion
This study describes the cleaning behavior of Mobula kuhlii for the first time in South Africa. Our results support the initial findings of Murie and Marshall (2016) in the Bazaruto Archipelago of southern Mozambique, confirming that this species actively cleans on shallow, inshore reefs in a similar manner to manta ray species. This is only the second aggregation site scientifically documented where individual M. kuhlii receive cleaning services discovered after Two Mile Reef, Mozambique. Both mature male and pregnant female M. kuhlii were observed cleaning at Aliwal Shoal MPA, and this site was consistently utilized over several years. These results indicate that the Aliwal Shoal MPA to be important habitat for M. kuhlii due to their repeated use of the specific cleaning station and for the occurrence of mating at this area (Carpenter and Griffiths 2023). This easily-accessible site also presents an excellent opportunity to continually monitor this species, which is Endangered and currently unprotected in South Africa.
Mobula kuhlii were significantly more likely to be encountered at sea surface temperatures of 24–26 °C, which is normal for summer (December-January) in KZN (Smit et al. 2013), agreeing with other studies on mobulids (manta rays, M. alfredi, M. birostris) in this area (Carpenter et al. 2023). Summer in KZN is known for increased productivity and subsequent abundance of zooplankton (Lamont and Barlow 2015). A group of > 150 M. kuhlii was encountered in December 2021, with many of these individuals exhibiting feeding behavior, confirming that the reef within the MPA supports both feeding and cleaning sites. While M. kuhii were encountered at all recreationally-visited dive sites, individuals predominantly cleaned at Angel’s Ledge and nearby locations, including Bay of Caves and Kyles Reef. The majority of cleaning activity took place along a very specific section of the reef, supporting findings of other mobulid studies, which found that individuals were highly selective and showed affinity to specific sites for cleaning (Couturier et al. 2012; Murie and Marshall 2016).
Unlike manta rays, which support cleaning from a variety of cleaner species (Marshall 2008), only L. dimidiatus were recorded cleaning M. kuhlii, with all body patches being cleaned, and a significant preference for the pelvic fins, dorsal body, and ventral head surfaces. Our results on pelvic fin preference partially agree (pelvic fins) with a study on Mobula birostris which was selectively foraged by L. dimidiatus on the gills, pelvic fins, and pectoral fins (Murie et al. 2020). Different cleaner fish have been documented to clean different parts of large-bodied clients, with certain fish species targeting specific parasites (Marshall 2008; Murie et al. 2020). Labroides dimidiatus is known to preferentially target caligid copepods (Pupulina cliffi) (Grutter 1997) and in KZN, M. kuhlii has been found to have high density loads of this ectoparasite (Lebepe and Dippenaar 2013). Parasitic copepods can impact the growth, fecundity, and survival of their wild hosts by feeding on mucous, tissues, and blood (Neilson et al. 1987; Johnson et al. 1996; Clague et al. 2011). The attachment and feeding activities by parasitic copepods lead to primary diseases, skin lesions, and secondary infections in host species (Neilson et al. 1987; Johnson et al. 1996, 2004; Ho et al. 2000). Thus, visiting cleaning stations, such as those found on Aliwal Shoal MPA may be important for M. kuhlii health.
Our findings support other studies on mobulids that show individuals feeding, cleaning and exhibiting courtship in close proximity (Stewart et al. 2016; Stevens et al 2018; Germanov et al. 2019). Often, environmental conditions correspond with each activity (Rohner et al. 2013). In this study, M. kuhlii cleaned for significantly longer periods during North currents, which is often associated with the presence of the warmer, nutrient-poor Agulhas current (Hutchings et al. 2002). Contrastingly, South currents are associated with colder water and green, more turbid visibility, which may be more productive. These collective findings may be indicative of the tendency of M. kuhlii to engage in cleaning activities when it is suboptimal for them to feed (Barr and Abelson 2019). Notably, Aliwal Shoal is influenced by various water bodies that affect zooplankton abundance (Pretorius et al. 2016). As such, M. kuhlii might exhibit a trade-off between cleaning and feeding, foraging when conditions are favorable with high abundances of plankton and cleaning when food densities are low (Barr and Abelson 2019; Murie et al. 2020).
Our findings suggest that M. kuhlii cleaning behavior is similar to that of other mobulids, including M. alfredi. M. kuhlii rarely cleaned alone, being in close proximity (1 m or less) to at least one conspecific individual 87% of the time recorded (n = 4,395 s). Mobulids are known to be social elasmobranchs (Notarbartolo di Sciara 1987; Perryman et al. 2019, 2022b) and Kitchen-Wheeler (2013) hypothesized that the presence of an established M. alfredi individual at a cleaning station may bring in other individuals, who then imitate their behavior. Mobula alfredi are well-studied for their sociality, and are thought to use cleaning stations as ‘social meeting points’ where they interact with preferred social partners (Perryman et al. 2019, 2022b). They also exhibit ‘following’ behavior (Perryman et al. 2021), reflecting a possible learned ritual. Further, the distinct contrasting dark and light colouration on the cephalic lobes of Mobula spp. has been suggested to aid in social signaling (Notarbartolo di Sciara 1987), which may occur at M. alfredi cleaning stations (Perryman et al. 2021). Mobula kuhlii recorded in this study displayed similar cephalic lobe positions to M. alfredi (Perryman et al. 2021), as well as similar ‘posing’ positions while cleaning (Marshall 2008). However, more research on M. kuhlii cleaning behavior is needed to determine the detailed social behavior patterns associated with these areas.
Mobula kuhlii occasionally visited cleaning stations at the same time as other megafauna species (7% of the time), but never with another mobulid species. Manta rays (M. alfredi, M. birostris) have been seen cleaning at the Pinnacles dive site on the Aliwal Shoal MPA and spotted ragged tooth sharks, Carcharias taurus, have been seen cleaning at the dive sites Cathedral and Chunnel (Carpenter, pers. obs.). Manta rays and ragged tooth sharks all have a larger total length or disc width than M. kuhlii. Many cleaning studies have reported on larger individuals being preferred by cleaner fish, and this is attributed to them likely having more parasites on a greater surface area (Grutter et al. 2005; Oliver et al. 2011; Kitchen-Wheeler 2013). Likewise, in Vilanculos, Mozambique, there is no observed instance of M. alfredi engaging in cleaning activities at Two Mile Reef, which is the originally-discovered M. kuhlii cleaning station (Murie and Marshall 2016). Moreover, no sightings of M. kuhlii have been recorded at a well-documented M. alfredi cleaning station in southern Mozambique, despite more than two decades of research efforts in the region (Marshall, pers. comm.). A total of 57% of the time that M. kuhlii cleaned with another megafauna species, this was with one or more A. ocellatus, the first description of these species simultaneously cleaning at the same cleaning station. It is possible that the cleaning station habitat was partitioned by M. kuhlii to avoid competition with larger, more dominant species, with adult A. ocellatus being only slightly larger or the same size (White et al. 2010) as adult M. kuhlii.
Mobula kuhlii is currently unprotected in South Africa. In KZN, gill netting as a means of subsistence is not prolific, but bather protective nets are gill nets that sometimes catch mobulids (Young 2001). As Aliwal Shoal MPA is a heavily-used recreational area, it is crucial that unregulated scuba and snorkeling tourism does not further impact M. kuhlii cleaning behavior, as it has been shown to affect M. alfredi (Kitchen-Wheeler 2013; Venables et al. 2016). All M. kuhlii seen on Aliwal Shoal MPA were of adult size, and included mature males and pregnant females, supporting the importance of this area for reproduction (Carpenter and Griffiths 2023). Reproductive behavior may also be negatively impacted by recreational diving if divers interrupt the behavior (Venables 2013; Murray et al. 2020). Therefore, we recommend a strict code of conduct procedure at the Aliwal Shoal MPA, which has already been implemented at the local dive centers by the authors. We also recommend this species to be awarded national protection due to its conservation status and the potential importance to the ecotourism economy (Russel 2022, Dive Magazine).
In this study, we emphasize the importance of understanding fine-scale habitat use in the southernmost range of M. kuhlii distribution. Our findings support the role of cleaning stations in mobulids for social and reproductive purposes. The aggregation site's location offers unique opportunities for ecotourism and long-term monitoring of Endangered M. kuhlii. To improve future studies at this site, employing high-definition video recording devices could capture and analyze complex interactions more effectively through slow-motion reviews, or even 360° camera systems for broader fields of view. Additionally, future investigations should focus on delineating juvenile habitats for M. kuhlii, incorporating acoustic and/or satellite telemetry to verify their movements within and outside the MPA. A precise understanding of movement patterns and the use of critical inshore habitats is crucial for effectively safeguarding this endangered species. Considering their vulnerability and the potential for ecotourism, prioritizing national protection measures and management plans for M. kuhlii in South Africa is imperative.
Data availability
The datasets generated and/or analyzed during the study are available from the corresponding author on reasonable request.
References
Araujo G, Miranda JA, Allen HL, Labaja J, Snow S, Ponzo A, Legaspi CG (2020) Whale sharks rhincodon typus get cleaned by the blue-streak cleaner wrasse Labroides dimidiatus and the moon wrasse Thalassoma lunare in the Philippines. J Fish Biol 97(4):1247–1251. https://doi.org/10.1111/jfb.14464
Barr Y, Abelson A (2019) Feeding–cleaning trade-off: manta ray “decision-making” as a conservation tool. Front Mar Sci 6:88. https://doi.org/10.3389/fmars.2019.00088
Bosman C, Uken R, Ovechkina MN (2007) The aliwal shoal revisited: new age constraints from nannofossil assemblages. S Afr J Geol 110(4):647–653. https://doi.org/10.2113/gssajg.110.4.647
Bosman C, Uken R, Smith AM (2005) The bathymetry of the Aliwal Shoal, Scottburgh, South Africa. S Afr J Sci 101:255–257. https://hdl.handle.net/10520/EJC96392.
Bshary R, Grutter AS (2002) Experimental evidence that partner choice is a driving force in the payoff distribution among cooperators or mutualists: the cleaner fish case. Ecol Lett 5(1):130–136. https://doi.org/10.1046/j.1461-0248.2002.00295.x
Caira JN, Healy CJ (2004) Elasmobranchs as hosts of metazoan parasites. In: Press CRC (ed) Lutz PL, Biology of Sharks and their Relatives. Florida, Boca Raton, pp 523–551
Carpenter M, Parker D, Dicken ML, Griffiths CL (2023) Multi-decade catches of manta rays (Mobula alfredi, M. birostris) from South Africa reveal significant decline. Front Mar Sci 29(10):1128819
Carpenter M, Griffiths C (2023) ‘Flash Mobula’: first observations of courtship behavior of the shortfin devil ray Mobula kuhlii. Afr J Mar Sci 45(1):51–56. https://doi.org/10.2989/1814232X.2022.2158131
Clague GE, Cheney KL, Goldizen AW, McCormick MI, Waldie PA, Grutter AS (2011) Long-term cleaner fish presence affects growth of a coral reef fish. Biol Lett 7(6):863–865. https://doi.org/10.1098/rsbl.2011.0458
Couturier LI, Marshall AD, Jaine FR, Kashiwagi T, Pierce SJ, Townsend KA, Weeks SJ, Bennett MB, Richardson AJ (2012) Biology, ecology and conservation of the Mobulidae. J Fish Biol 80(5):1075–1119. https://doi.org/10.1111/j.1095-8649.2012.03264.x
Croll DA, Dewar H, Dulvy NK, Fernando D, Francis MP, Galván-Magaña F, Hall M, Heinrichs S, Marshall A, Mccauley D, Newton KM (2016) Vulnerabilities and fisheries impacts: the uncertain future of manta and devil rays. Aquat Conserv 26(3):562–575. https://doi.org/10.1002/aqc.2591
Deakos MH, Baker JD, Bejder L (2011) Characteristics of a manta ray manta alfredi population off maui, hawaii, and implications for management. Mar Ecol Prog Ser 429:245–260. https://doi.org/10.3354/meps09085
Dicken ML, Hosking SG (2009) Socio-economic aspects of the tiger shark diving industry within the aliwal shoal marine protected area. S Afr Afr J Mar Sci 31(2):227–232. https://doi.org/10.2989/AJMS.2009.31.2.10.882
Dicken ML, Smale MJ, Booth AJ (2006) Spatial and seasonal distribution patterns of the ragged-tooth shark Carcharias taurus along the coast of South Africa. Afr J Mar Sci 28:603–616. https://doi.org/10.2989/18142320609504210
Dudgeon CL, Lanyon JM, Semmens JM (2013) Seasonality and site fidelity of the zebra shark, Stegostoma fasciatum, in southeast Queensland. Aust Anim Behav 85(2):471–481. https://doi.org/10.1016/j.anbehav.2012.12.013
Dulvy NK, Pardo SA, Simpfendorfer CA, Carlson JK (2014) Diagnosing the dangerous demography of manta rays using life history theory. PeerJ 2:e400
Dunn OJ (1961) Multiple comparisons among means. J Am Stat Assoc 56(293):52–64. https://doi.org/10.1080/01621459.1961.10482090
Feder HM (1966) Cleaning symbiosis in the marine environment. Symbiosis 1:327–380
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7(11):1325–1330. https://doi.org/10.1111/2041-210X.12584
Germanov ES, Bejder L, Chabanne DB, Dharmadi D, Hendrawan IG, Marshall AD, Pierce SJ, van Keulen M, Loneragan NR (2019) Contrasting habitat use and population dynamics of reef manta rays within the nusa penida marine protected area. Indones Front Mar Sci 6:215. https://doi.org/10.3389/fmars.2019.00215
Grutter AS, Glover S, Bshary R (2005) Does client size affect cleaner fish choice of client? an empirical test using client fish models. J Fish Biol 66(6):1748–1752. https://doi.org/10.1111/j.1095-8649.2005.00709.x
Grutter AS (1997) Spatiotemporal variation and feeding selectivity in the diet of the cleaner fish. Labroides Dimidiatus Copeia. https://doi.org/10.2307/1447754
Hearn A, Ketchum J, Klimley AP, Espinoza E, Penaherrera C (2010) Hotspots within hotspots? Hammerhead shark movements around Wolf Island. Galapagos Marine Reserve Mar Biol 157(9):1899–1915. https://doi.org/10.1007/s00227-010-1460-2
Heydorn AE, Bang ND, Pearce AF, Flemming BW, Carter RA, Schleyer MH, Berry PF, Hughes GR, Bass AJ, Wallace JH, Van Der Elst RP (1978) Ecology of the Agulhas Current region: an assessment of biological responses to environmental parameters in the south-west Indian Ocean. Trans Royal Soc S Afr 43(2):151–190. https://doi.org/10.1080/00359197809520235
Ho JS, Lin CL, Chen SN (2000) Species of Caligus Muller, 1785 (Copepoda: Caligidae) parasitic on marine fishes of Taiwan. Syst Parasitol 46:159–179
Hutchings L, Beckley LE, Griffiths MH, Roberts MJ, Sundby S, Van der Lingen C (2002) Spawning on the edge: spawning grounds and nursery areas around the southern African coastline. Mar Freshw Res 53(2):307–318. https://doi.org/10.1071/MF01147
Johnson SC, Blaylock RB, Elphick J, Hyatt KD (1996) Disease induced by the sea louse (Lepeophteirus salmonis) (Copepoda: Caligidae) in wild sockeye salmon (Oncorhynchus nerka) stocks of Alberni Inlet, British Columbia. Can J Fish Aquat Sci 12:2888–2897
Johnson SC, Bravo S, Nagasawa K, Kabata Z, Hwang J, Ho J, Shih CT (2004) A review of the impact of parasitic copepods on marine aquaculture. Zool Studies 1(43):229–243
Keyes RS (1982) Sharks: an unusual example of cleaning symbiosis. Copeia 1:225–227. https://doi.org/10.2307/1444305
Kitchen-Wheeler AM (2013) The behavior and ecology of Alfred mantas (Manta alfredi) in the Maldives. PhD thesis, Newcastle University, School of Biological Sciences
Lamont T, Barlow RG (2015) Environmental influence on phytoplankton production during summer on the KwaZulu-Natal shelf of the Agulhas ecosystem. Afr J Mar Sci 37:485–501
Lebepe MC, Dippenaar SM (2013) A report of symbiotic Siphonostomatoida (Copepoda) infecting mobulids (Rajiformes: Mobulidae) off the KwaZulu-Natal coast, South Africa. Afr Zool 48:326–332. https://doi.org/10.1080/15627020.2013.11407599
Limbaugh C (1961) Clean Symbiosis Sci Amer 205(2):42–49
Losey GS Jr (1972) The ecological importance of cleaning symbiosis. Copeia 4:820–833. https://doi.org/10.2307/1442741
Marshall AD (2008) Biology and population ecology of Manta birostris in southern Mozambique. PhD thesis, University of Queensland, School of Biomedical Sciences
Marshall AD, Bennett MB (2010a) Reproductive ecology of the reef manta ray Manta alfredi in southern Mozambique. J Fish Biol 77(1):169–190. https://doi.org/10.1111/j.1095-8649.2010.02669.x
Marshall AD, Bennett MB (2010b) The frequency and effect of shark-inflicted bite injuries to the reef manta ray Manta alfredi. Afr J Mar Sci 32(3):573–580. https://doi.org/10.2989/1814232X.2010.538152
Meekan MG, Trevitt L, Simpfendorfer CA, White W (2016) The piggybacking stingray. Coral Reefs 35(3):1011. https://doi.org/10.1007/s00338-016-1429-9
Murie C, Spencer M, Oliver SP (2020) Current strength, temperature, and bodyscape modulate cleaning services for giant manta rays. Mar Biol 167(5):1–11. https://doi.org/10.1007/s00227-020-3674-2
Murie CJ, Marshall AD (2016) Mobula kuhlii cleaning station identified at an inshore reef in southern Mozambique. PeerJ PrePrints. https://doi.org/10.7287/peerj.preprints.1724v1
Murray A, Garrud E, Ender I, Lee-Brooks K, Atkins R, Lynam R, Arnold K, Roberts C, Hawkins J, Stevens G (2020) Protecting the million-dollar mantas; creating an evidence-based code of conduct for manta ray tourism interactions. J Ecotourism 19:132–147. https://doi.org/10.1080/14724049.2019.1659802
Neilson JD, Perry RI, Scott JS, Valerio P (1987) Interactions of caligid ectoparasites and juvenile gadids on Georges Bank. Mar Ecol Prog Ser 39(3):221–232
Notarbartolo di Sciara G (1987) A revisionary study of the genus Mobula Rafinesque, 1810 Chondrichthyes: Mobulidae with the description of a new species. Zool J Linn Soc. https://doi.org/10.1111/j.1096-3642.1987.tb01723.x
O’Shea OR, Kingsford MJ, Seymour J (2010) Tide-related periodicity of manta rays and sharks to cleaning stations on a coral reef. Mar Freshw Res 61(1):65–73. https://doi.org/10.1071/MF08301
Olbers JM, Celliers L, Schleyer MH (2009) Zonation of benthic communities on the subtropical Aliwal Shoal, Durban, KwaZulu-Natal. South Africa Afr Zoo 44(1):8–23. https://doi.org/10.1080/15627020.2009.11407435
Oliver SP, Kaszo AB (2015) A pelagic thresher shark (Alopias pelagicus) gives birth at a cleaning station in the Philippines. Coral Reefs 34(1):17–17. https://doi.org/10.1007/s00338-014-1249-8
Oliver SP, Hussey NE, Turner JR, Beckett AJ (2011) Oceanic sharks clean at coastal seamount. PLoS ONE 6(3):e14755. https://doi.org/10.1371/journal.pone.0014755
Perryman RJ, Venables SK, Tapilatu RF, Marshall AD, Brown C, Franks DW (2019) Social preferences and network structure in a population of reef manta rays. Behav Ecol Socbiol 73(8):1–8. https://doi.org/10.1007/s00265-019-2720-x
Perryman RJ, Carpenter M, Lie E, Sofronov G, Marshall AD, Brown C (2021) Reef manta ray cephalic lobe movements are modulated during social interactions. Behav Ecol Sociobiol 75:1–5. https://doi.org/10.1007/s00265-021-02973-x
Perryman RJ, Mourier J, Venables SK, Tapilatu RF, Setyawan E, Brown C (2022b) Reef manta ray social dynamics depend on individual differences in behavior. Anim Behav 191:43–55. https://doi.org/10.1016/j.anbehav.2022.06.010
Perryman RJ, Brown C, Pasian N, Ward AJ, Kent MI (2022a) Investigating manta ray collective movements via drone surveys. bioRxiv. 03:06–393
Pierce SJ, White WT, Marshall AD (2008) New record of the smalleye stingray Dasyatis microps Myliobatiformes: Dasyatidae from the western Indian Ocean. Zootaxa. https://doi.org/10.11646/zootaxa.1734.1.5
Pretorius M, Huggett JA, Gibbons MJ (2016) Summer and winter differences in zooplankton biomass, distribution and size composition in the KwaZulu-Natal Bight, South Africa. Afr J Mar Sci 38:155–168
Quimbayo JP, Dias MS, Schlickmann OR, Mendes TC (2017a) Fish cleaning interactions on a remote island in the Tropical Eastern Pacific. Mar Biodiv 47(2):603–608. https://doi.org/10.1007/s12526-016-0493-2
Quimbayo JP, Nunes LT, Ozekoski R, Floeter SR, Morais RA, Fontoura L, Bonaldo RM, Ferreira CE, Sazima I (2017b) Cleaning interactions at the only atoll in the South Atlantic. Environ Biol Fishes 100(7):865–875. https://doi.org/10.1007/s10641-017-0612-3
Rigby CL, Barreto R, Carlson J, Fernando D, Fordham S, Francis MP, Jabado RW, Liu KM, Marshall A, Romanov E (2022) Mobula kuhlii (amended version of 2020 assessment). IUCN Red List Threat Speci. https://doi.org/10.2305/IUCN.UK.2022-1.RLTS.T161439A214405747.en
Rohner CA, Pierce SJ, Marshall AD, Weeks SJ, Bennett MB, Richardson AJ (2013) Trends in sightings and environmental influences on a coastal aggregation of manta rays and whale sharks. Mar Ecol Prog Ser 482:153–168. https://doi.org/10.3354/meps10290
Rohner CA, Flam AL, Pierce SJ, Marshall AD (2017) Steep declines in sightings of manta rays and devilrays Mobulidae in southern Mozambique. PeerJ Preprints. 5:e3051v1. https://doi.org/10.7287/peerj.preprints.3051v1
Ros AF, Nusbaumer D, Triki Z, Grutter AS, Bshary R (2020) The impact of long-term reduced access to cleaner fish on health indicators of resident client fish. J Exp Biol 223(24):jeb231613
Russel MC (2022) Devil Ray Conservation Dive launched at Aliwal Shoal. Dive Magazine, https://divemagazine.com/scuba-diving-news/devil-ray-conservation-dive-launched-at-aliwal-shoal.
Sazima I, Moura RL (2000) Shark (Carcharhinus perezi), cleaned by the goby (Elacatinus randalli), at Fernando de Noronha Archipelago, western South Atlantic. Copeia 1:297–299. https://doi.org/10.1643/0045-8511(2000)2000[0297:SCPCBT]2.0.CO;2
Smit AJ, Roberts M, Anderson RJ, Dufois F, Dudley SF, Bornman TG, Olbers J, Bolton JJ (2013) A coastal seawater temperature dataset for biogeographical studies: large biases between in situ and remotely-sensed data sets around the coast of South Africa. PLoS One 8(12):e81944. https://doi.org/10.1371/journal.pone.0081944
Snelson FF Jr, Gruber SH, Murru FL (1990) Schmid TH (1990) Southern stingray, Dasyatis americana: host for a symbiotic cleaner wrasse. Copeia 4:961–965. https://doi.org/10.2307/1446479
Soares MC, Bshary R, Cardoso SC, Côté IM (2008) The meaning of jolts by fish clients of cleaning gobies. J Ethol 114(3):209–214. https://doi.org/10.1111/j.1439-0310.2007.01471.x
Stevens GMW (2016) Conservation and population ecology of manta rays in the Maldives. PhD thesis, University of York, School of Environment
Stevens GM, Hawkins JP, Roberts CM (2018) Courtship and mating behavior of manta rays Mobula alfredi and M. birostris in the Maldives. J Fish Biol 93(2):344–359. https://doi.org/10.1111/jfb.13768
Stewart JD, Beale CS, Fernando D, Sianipar AB, Burton RS, Semmens BX, Aburto-Oropeza O (2016) Spatial ecology and conservation of Manta birostris in the Indo-Pacific. Biol Conserv 200:178–183. https://doi.org/10.1016/j.biocon.2016.05.016
Stewart JD, Jaine FR, Armstrong AJ, Armstrong AO, Bennett MB, Burgess KB, Couturier LI, Croll DA, Cronin MR, Deakos MH, Dudgeon CL (2018) Research priorities to support effective manta and devil ray conservation. Front Mar Sci 5:314. https://doi.org/10.3389/fmars.2018.00314
Thieurmel B, Elmarhraoui A (2019) Suncalc: Compute Sun Position, Sunlight Phases, Moon Position and Lunar Phase. R package version 0.5.0. https://CRAN.R-project.org/package=suncalc.
Venables S (2013) Short term behavioural responses of manta rays, Manta alfredi, to tourism interactions in Coral Bay, Western Australia. Honours dissertation, Murdoch University
Venables S, McGregor F, Brain L, van Keulen M (2016) Manta ray tourism management, precautionary strategies for a growing industry: a case study from the Ningaloo Marine Park. Western Australia Pac Conserv Biol 22(4):295–300. https://doi.org/10.1071/PC16003
Wheeler S, Robbins WD, McIllwain J (2013) Reef sharks clean up with a novel inshore mutualistic interaction. Coral Reefs 32(4):1089. https://doi.org/10.1007/s00338-013-1068-3
White WT, Giles J, Potter IC (2006) Data on the bycatch fishery and reproductive biology of mobulid rays (Myliobatiformes) in Indonesia. Fish Res 82(1–3):65–73. https://doi.org/10.1016/j.fishres.2006.08.008
Whitney NM, Motta PJ (2008) Cleaner host posing behavior of whitetip reef sharks (Triaenodon obesus) in a swarm of hyperiid amphipods. Coral Reefs 27(2):363. https://doi.org/10.1007/s00338-007-0345-4
Wickham H (2016) Ggplot2: Elegant graphics for data analysis, 2nd edn. Springer International Publishing
Wood, SN (2006) The mgcv package. R statistical group.
Young N (2001) An analysis of the trends in by-catch of turtle species, angelsharks, and batoid species in the protective gillnets off KwaZulu-Natal, South Africa. MSc Thesis, University of Reading, School of Biological Sciences Reading.
Acknowledgements
This work would not have been possible without the financial support of the University of Cape Town and the Rufford Foundation. We are grateful to Olivia Symcox of Olivia Jones Communications, Mohammed Kajee, Marine Megafauna Foundation, and Kent Taylor, for their time, assistance, and in-kind support during the project. We specifically acknowledge ScubaXcursion Dive Centre, Freediving South Africa, Blue Ocean Dive Centre, ScubaCo Dive and Travel, and Agulhas Dive Centre for their recreational diving / snorkeling support. We thank the reviewers for their helpful feedback which improved the manuscript.
Funding
Open access funding provided by University of Cape Town. This work was funded by the Rufford Foundation and University of Cape Town. The funding body did not influence the design of the study and collection, analysis, and interpretation of the data nor the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
MC and AM conceived the idea of the study. Data was collected by MC. Data analysis was conducted by MC and RP. MC wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
No ethical approval was required.
Additional information
Responsible Editor: J. Carlson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carpenter, M.M., Perryman, R.J.Y., Marshall, A.D. et al. Behavior, site use and demographics of shortfin devil rays, Mobula kuhlii, at a newly-discovered cleaning area in South Africa. Mar Biol 171, 130 (2024). https://doi.org/10.1007/s00227-024-04444-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04444-z